23.2: Model Building
- Page ID
- 41059
An overarching goal of metabolic modeling is the ability to take a schematic representation of a pathway and change that it into a mathematical formula modeling the pathway. For example, converting the following pathway into a mathematical model would be incredible useful.
Chemical Reactions
In metabolic models, we are concerned with modeling chemical reactions that are catalyzed by enzymes. Enzymes work by acting on a transition state of the enzyme-substrate complex that lowers the activation energy of a chemical reaction. The diagram on slide 5 of page 1 of the lecture slides demonstrates this phenomenon. A typical rate equation (which describes the conversion of the substrates S of the enzyme reaction into its products P) can be described by a Michaelis-Menten rate law:
\[\frac{V}{V_{\max }}=\frac{[S]}{K_{\mathrm{m}}+[S]}\nonumber\]
In this equation, V is the rate of the equation as a function of substrate concentration [S]. It is clear that the parameters Km and Vmax are necessary to characterize the equation.
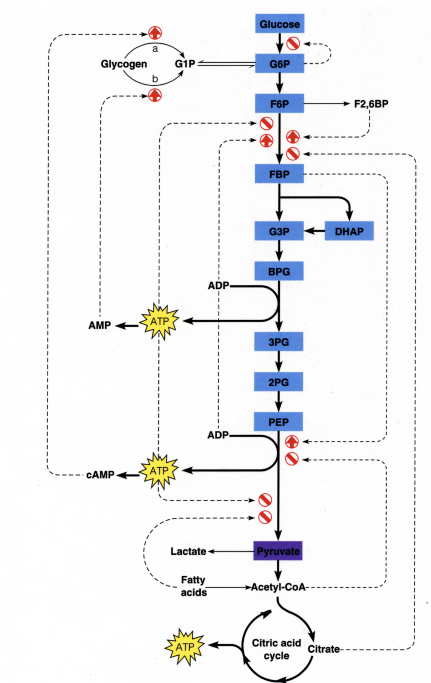
The inclusion of multiple substrates, products, and regulatory relationships quickly increases the number of parameters necessary to characterize such equations. The figures on slides 1, 2, and 3 of page 2 of the lecture notes demonstrate the complexity of biochemical pathways. Kinetic modeling quickly becomes infeasible: the necessary parameters are dicult to measure, and also vary across organisms [10]. Thus, we are interested in a modeling method that would allow us to use a small number of precisely determined parameters. To this end, we recall the basic machinery of stoichiometry from general chemistry. Consider the chemical equation A+2B ! 3C, which says that one unit of reactant A combines with 2 units of reactant B to form 3 units of reactant C. The rate of formation of the compound X is given by the time derivative of [X]. Note that C forms three times as fast as A. Therefore, due to the stoichiometry of the reaction, we see that the reaction rate (or reaction flux) is given by
\[f l u x=\frac{d[A]}{d t}=\frac{1}{2} \frac{d[B]}{d t}=\frac{1}{3} \frac{d[C]}{d t} \nonumber \]
This will be useful in the subsequent sections. We must now state the simplifying assumptions that make our model tractable.
Steady-State Assumption
The steady state assumption assumes that there is no accumulation of any metabolite in the system. This allows us to represent reactions entirely in terms of their chemistry (i.e. the stoichiometric relationships between the components of the enzymatic reaction). Note that this does not imply the absence of flux through any given reaction. Rather, steady-state actually implies two assumptions that are critical to simplify metabolic modeling. The first is that the internal metabolite concentrations are constant, and the second is that fluxes, ie input and output fluxes, are also constant.
An analogy is a series of waterfalls that contribute water to pools. As the water falls from one pool to another, the water levels do not change even though water continues to flow (see page 2 slide 5). This framework prevents us from being hindered by the overly complicated transient kinetics that can result from perturbations of the system. Since we are usually interested in long-term metabolic capabilities (functions on a scale longer than milliseconds or seconds), the steady state dynamics may give us all the information that we need.
The steady-state assumption makes the ability to generalize across species and reuse conserved pathways in models much more feasible. Reaction stochiometries are often conserved across species, since they involve only conservation of mass. The biology of enzyme catalysis, and the parameters that characterize it, are not similarly conserved. These include species-dependent parameters such as the activation energy of a reaction, substrate anity of an enzyme, and the rate constants for various reactions. However, none of these are required for steady-state modeling.
It is also of interest to note that, since time constants for metabolic reactions are usually in the order of milliseconds, most measurement technologies used today are not able to capture these extremely fast dynamics. This is the case of metabolomics mass spectrometry based measurements for example. In this method, the amounts of all the internal metabolites in a system are measured at a given point in time, but measurements can be taken at best every hour. In the majority of circumstances, all that is ever measured is steady state.
Reconstructing Metabolic Pathways
There are several databases that can provide the information necessary to reconstruct metabolic pathways in silico. These databases allow reaction stoichiometry to be accessed using Enzyme Commission numbers. Reaction stochiometries are the same in all the organisms that utilize a given enzyme. Among the databases of interest are ExPASy [5], MetaCyc [16], and KEGG [14]. These databases often contain pathways organized by function that can be downloaded in SBML format, making pathway reconstruction very easy for well- characterized pathways.


