2.1: Using micropipettes correctly
- Page ID
- 17496
Arguably, the most important scientific equipment that you will use in this class are adjustable micropipettes, which you will use in nearly every experiment. Micropipettes are precision instruments that are designed to accurately and precisely transfer volumes in the microliter range. You may use microliters or milliliters as the units of volume in your lab notebooks and lab reports, but be careful to always state the volume unit that you are using. Recall the relationships between volume units:
Accuracy and precision
Accuracy depends on the micropipette delivering the correct volume. Precise results
are reproducible. Let’s use a target analogy to demonstrate the difference between accurate and precise results. Imagine that four students try to hit the bulls-eye five times. Students A and B are precise, while students A and C are accurate.
Manufacturers determine the accuracy and precision of micropipettes by using them to transfer defined volumes of distilled water to a drop that is then weighed on an analytical balance. The density of water is 1.0 gram per mL at 25 ̊C. The process is repeated several times during the calibration process, and the data is used to calculate the accuracy and precision of a micropipette.
Accuracy refers to the performance of the micropipette relative to a standard (the intended) value. Accuracy is computed from the difference between the actual volume dispensed by the micropipette and the intended volume. Note that this can be a negative or positive
value. When micropipettes are calibrated, the accuracy is normally expressed as a percent of
the selected value. Micropipettes are designed to operate with accuracies within a few percent (generally <3%) of the intended value. The accuracy of a micropipette decreases somewhat when micropipettes are set to deliver volumes close to the lowest values in their range.
Precision provides information about reproducibility, without any reference to a standard. Precision reflects random errors that can never be entirely eliminated from a procedure. Thus, a series of repeated measurements should generate a normal or binomial distribution (opposite). Precision is expressed as the standard deviation (s) of the set of measurements. In a normal distribution, ~2/3 of measurements will fall within one standard deviation of the average or mean (x), and 95% of measurements will fall within two standard deviations of the mean. The standard deviation for a set of n measurements is calculated using the formula below.
Choosing the micropipette
Standard deviation describes the distribution of measurments relative to the mean value
We use three different sizes of micropipettes in the laboratory, the P20, P200 and P1000. Our micropipettes have been purchased from several different manufacturers, but the principles of operation are the same. The numbers after the “P” refer to the maximum number of microli- ters that the micropipette is designed to transfer. Note that there is some overlap in the ranges of the different micropipettes. For example, both the P200 and P20 can be used to transfer 15 μl, but the P20 is more accurate within that range. As a rule of thumb, always select the smallest volume pipette that will transfer the volume.
Specifying the transfer volume
There are three numbers on the volume indicator. With each of the micropipettes, you will specify a volume to three digits by turning the volume adjustment knob. You will also be able to extrapolate between the lowest numbers with the vernier marks on the lower dial. Most of the measurements you will make with the micropipettes will be accurate to four significant figures!
NEVER turn the indicator dial beyond the upper or lower volume limits of the micropipette! This could damage the piston.
Transferring volumes accurately
Micropipettes work by air displacement. The operator depresses a plunger that moves an internal piston to one of two different positions. The first stop is used to fill the micropipette tip, and the second stop is used to dispense the contents of the tip. As the operator depresses the plunger to the first stop, an internal piston displaces a volume of air equal to the volume shown on the volume indicator dial. The second stop is used only to dispense the contents of the tip.
Filling the micropipette
- Remove the lid from the box containing the correct size micropipette tips. P-1000 tips may be blue or clear, while P-20 and P-200 tips are yellow or clear.
- Attach the tip by inserting the shaft of the micropipette into the tip and pressing down firmly (figure on right). This should produce an airtight seal between the tip and the shaft of the micropipette.
- Replace the lid of the tip box to keep the remaining tips sterile. Avoid touching the tip (especially the thinner end), because the tips are sterile.
- Depress the plunger of the micropipette to the FIRST stop.
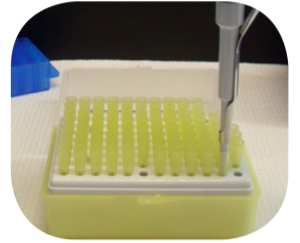
- Immerse the tip a few millimeters below the surface of the solution being drawn up into the
pipette. Pipetting is most accurate when the pipette is held vertically. Keep the angle less
than 20 ̊ from vertical for best results.
- Release the plunger S L O W L Y, allowing the tip to fill smoothly. Pause briefly to ensure
that the full volume of sample has entered the tip. Do NOT let the plunger snap up. This is particularly important when transferring larger volumes, because a splash could contami- nate the shaft of the micropipette. If you inadvertently contaminate the shaft, clean it imme- diately with a damp Kimwipe.
NEVER rest a micropipette with fluid in its tip on the bench!
Dispensing the contents of the micropipette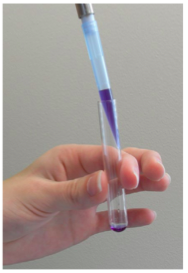
- Place the micropipette tip against the side of the receiving test tube. Surface tension will help to dispense the contents of the micropipette. Do NOT attempt to eject the contents of the micropipette into “thin air.”
- Smoothly depress the plunger to the first stop. Pause, then depress the plunger to the second stop. The contents of the pipette should have been largely released at the first stop. The second stop ensures that you’ve released the “last drop.”
- Use the tip ejector to discard the tip.


