7.7: Telomeres
- Page ID
- 17074
If there is a mechanism for recognizing loose ends of DNA, what about the ends of every eukaryotic chromosome? They are linear chromosomes, so they have ends, right? What prevents the double-strand-break repair systems from mis-recognizing them all as broken DNA and concatenating all of the chromosomes together? Interestingly, the answer to this question is intimately tied up with the answer to the problem of end- replication, which was very briefly alluded to in our description of replication.
The end-replication problem is one that affects all linear chromosomes. It boils down to one simple fact: an RNA primer is needed to start any DNA replication. So on the 5’ end of each strand is an RNA primer (Figure \(\PageIndex{24}\) in yellow) that gets removed by the error-correction process. Thus with every round of replication, information is lost from the 5’ end of each strand of each chromosome.

Eventually, crucial genes are lost and the cell will die; most likely many cellular functions will be compromised long before that happens. The solution to the end-replication problem might be considered more a treatment of symptoms than a cure, to use an analogy to medicine. In short, during the very early stages of an organism’s life, a lot of non-coding DNA is added onto the ends of the DNA so that as the cell and its progeny continue to reproduce, the nucleotides do not affect any functional genes. This process is catalyzed by the enzyme telomerase.
Telomerase is a large holoenzyme that acts as a reverse transcriptase, reading a self-contained RNA template to add on telomere sequence to the 3’ ends of linear chromosomes. Note that this does not add onto the 5’ ends - as mentioned above, there is nothing to be done about the 5’ ends directly. However, while telomerases are active, the 3’ ends can be extended, and thus when the 5’ end primer is removed, the sequence lost (forever) from that strand of DNA is only a telomeric repeat and not something more useful. The repeats are well conserved across eukaryotes, and almost completely conserved across mammalian species (shown in Figure \(\PageIndex{25}\)).

In metazoans, telomerase activity is high in the embryonic stages of life, but is virtually non-existent in adults except in cell types that must constantly proliferate (e.g. blood and epithelial cells). The activity of telomerase is primarily regulated by expression of the TERT gene (telomerase reverse transcriptase) although construction of the full telomerase also requires the expression of the TERC gene (the telomerase RNA, also abbreviated TR), and dyskerin. Roughly speaking, the number of telomeric repeats that are placed on a chromosome in early development determines the number of DNA replications and cell divisions that the cell can undergo before succumbing to apoptosis (programmed cell death). Experiments on cells in culture demonstrate a strong correlation between telomere length and longevity, and it is known that cells taken from people with the premature aging disease, progeria, have relatively short telomeres.
Conversely, cancer cells almost universally have unregulated expression of telomerase. Given that a defining characteristic of cancer cells is the ability to proliferate rapidly and indefinitely, turning telomerase back on is, not surprisingly, an important aspect of carcinogenesis. It is therefore a target for anti-cancer treatments; however, to date no telomerase-targeting therapies have proven effective.
Now that we know about telomeres, the question that started this section becomes even more problematic: with these repeated sequence overhangs, how are chromosomes prevented from connecting end-to-end through a double-strand repair-like process? In part due to their repeated sequences, telomeres are able to form end-caps and protect chromosomal ends. The telomeres protect the ends of each chromosome by binding to protective proteins and by forming complex structures. Telomere end binding proteins (TEBP) bind to the 3’ overhanging end of the telomere. Other capping proteins, such as the mammalian TRF1 and TRF2 (telomere repeat binding factors) not only bind the telomere, but help to organize it into large looped structures known as T-loops (Figure \(\PageIndex{26}\)).
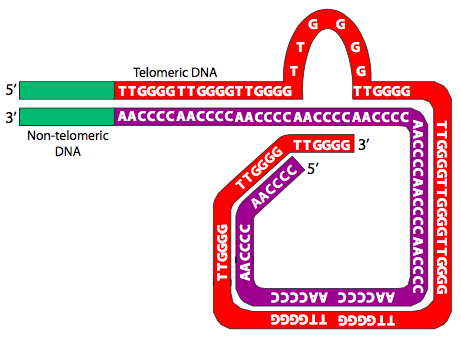
Finally, the T-loop ends are further stabilized by the formation of G-quartets (Figure \(\PageIndex{27}\)). G-quartets are a cyclic tetramers that can form in sequences with four consecutive guanine residues, which hydrogen-bond to each other to make a linked square shape stabilized by a metal ion in the center. Furthermore in cases like the telomere, in which such sequences are repeated, the G-quartets can stack and associate three-dimensionally, increasing their stability.


