3.4: Regulation of Enzyme Activity
- Page ID
- 16103
Figure \(\PageIndex{7}\) (and 9) also illustrates the effects of two different types of inhibition on the different components of enzyme kinetics. Enzymes can be slowed down or even prevented from catalyzing reactions in many ways including preventing the substrate from entering the active site or preventing the enzyme from altering conformation to catalyze the reaction. The inhibitors that do this can do so either reversibly or irreversibly. The irreversible inhibitors are also called inactivators, and either bind to the enzyme with such high afinity as to be virtually irreversible, or they actually form covalent bonds with the enzyme. Reversible inhibitors are generally grouped into two basic types: competitive and non-competitive.
Finasteride (trade names include Propecia and Proscar) is an irreversible inhibitor that binds very tightly to the enzyme 5-a-re- ductase, used in converting testosterone to dihydrotestosterone. It is used in the treatment of male pattern baldness, benign pro- static hyperplasia, and prostate cancer.
Aspirin is an example of an irreversible inhibitor that actually forms a covalent bond with the enzyme. The aspirin (acetylsalicylic acid) transfers its acetyl group onto a serine residue on cyclooxygenase-2 (COX-2). This stops the production of in ammation-producing prostaglandins and thromboxanes by COX-2.
Methotrexate is a competitive inhibitor of dihydrofolate reductase (DHFR), an enzyme that synthesizes tetrahydrofolate, which is a precursor for purine synthesis, and therefore for DNA and RNA. It has a very similar molecular structure for folic acid, the natural substrate of DHFR. Methotrexate is used as an anti-cancer drug because it affects rapidly reproducing cells (which need to make DNA sooner than other cells) more than non-cancerous cells.
Competitive inhibition is perhaps the simplest to understand. The inhibitor molecule competes directly with the substrate for the active site of an unbound enzyme. If an inhibitor binds to the active site, the substrate is unable to do so until the inhibitor has vacated the site. Thus, one could potentially overwhelm competitive inhibition with sufficiently larger concentrations of substrate so that the probability that the enzyme bumps into a substrate to bind becomes exceeding large compared to the probability of bumping into an inhibitor. Normal, uninhibited Vmax is then achieved despite the presence of the competitive inhibitor, which has only affected the Km, that is, the concentration of substrate needed to reach Vmax/2. This is the kinetic signature of competitive inhibitors: with increasing inhibitor concentrations, KM is increased but Vmax is unaffected.
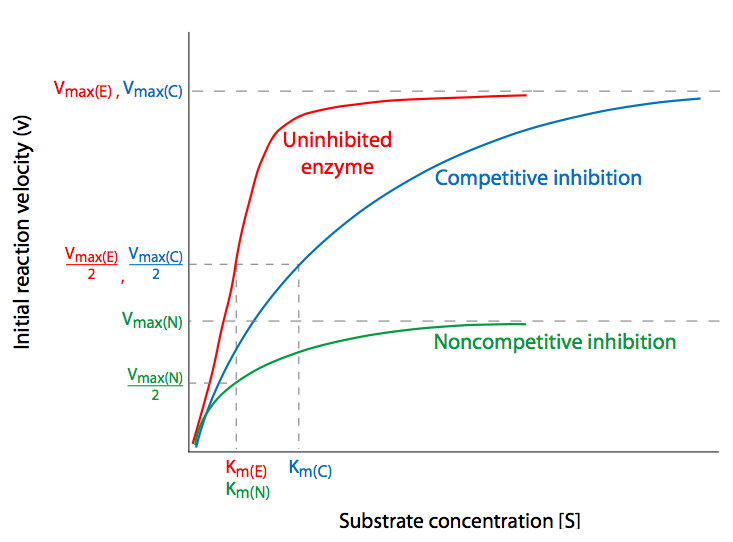
Non-competitive inhibition involves inhibiting the enzyme by altering its ability to complete the catalyzed reaction through binding of the enzyme at a position that is not the active site. When the inhibitor binds to the enzyme, it causes a change, usually con- formational, that may either prevent the enzyme from binding the substrate, or prevent the enzyme from acting upon a bound substrate. In either case, increasing the availability of substrate will not ultimately overcome the effect of the inhibitor. Thus, \(V_{max}\) is reduced not because some proportion of the enzymes are no longer usable, but because the enzymes that are available have the same access to substrate as it would without inhibitor (that is, it is not in competition with an inhibitor), the \(K_m\) is not affected.
Allostery
Non-competitive regulation is one example of allosteric regulation of enzymes. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a protein influences the binding of another ligand to the protein at a separate binding site. These kinds of interactions can be either positive (activating) or negative (inhibitory), and either homotropic (both ligands are identical) or heterotropic (ligands are different). Interestingly, sometimes the regulator ligand may actually be a product of the catalyzed reaction. In this kind of feedback mechanism, the progress of a reaction is self-regulating.
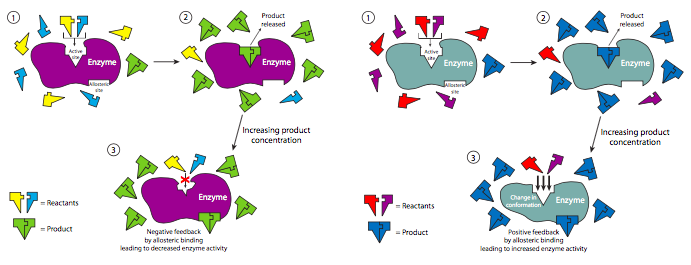
Many of the enzymes in metabolic pathways (chapters 5 and 6) are regulated by a naturally occurring non-competitive inhibitor. One example is phosphofructokinase (PFK), which is involved in glycolysis, which produces ATP for the cell. However, if there are high enough levels of ATP in the cell that other cellular processes aren’t using, then it can bind to phosphofructokinase outside of its active site (it binds fructose-6-phosphate) and turn it off. This blocks glycolysis and production of excess ATP when the cell does not need it. As ATP is used up, there is less available to inhibit PFK and glycolysis starts back up.
There are two models for such allosteric interactions.
- The symmetry model, also known as the concerted model, or MWC model (Monod, Wyman, and Changeux, 1965), proposes that the allosteric enzyme is an oligomer of several subunits, each of which are symmetrically related, and can be in either a “tensed” or “relaxed” state, but all of the subunits are in the same state and at equilibrium. When a ligand binds, it changes the state of the subunit(s) to which it binds, and to maintain equilibrium, that in turn causes the state of the other subunits to match, thus altering binding properties for a subsequent ligand.
- The sequential model, or the KNF model (Koshland, Nemethy, and Filmer, 1966), proposes something quite different: although it also supposes subunits in either tensed or relaxed states, it does not require all subunits to be connected in such a way as to mandate that all subunits be in the same state, and therefore conformational changes in one subunit need not be propagated to all. Instead, using an induced-fit model of ligand binding rather than the more rigid basic lock and key mechanism, it suggests that when a ligand binds to the enzyme, it induces a slight conformational change in the active site that increases its affinity for the ligand. The conformational change may slightly alter the conformation of other subunits of the enzyme, but may not constitute a state change between relaxed and tensed depending on how tightly the subunits are interacting. However, the change is enough to increase substrate affinity in adjacent subunits.
Most cell/molecular courses stop the discussion of enzyme inhibitors at competitive vs non-competitive based on their kinetic profiles. However, it should be noted that if you take a biochemistry course, you may encounter the terms uncompetitive inhibitor and mixed inhibitor. These terms are defined not just by the enzyme kinetics, but the mechanism of interaction:
- Uncompetitive inhibitors bind only to the enzyme-substrate (ES) complex, and not to the enzyme before it has encountered substrate. This leads to decreased Vmax and decreased Km.
- Mixed inhibition means that the inhibitor can bind to either enzyme alone or the enzyme-substrate complex. Because the affinities of the inhibitor for the two forms of the enzyme are different, and because part of it depends on substrate concentration while the other kind of binding does not, generally, Vmax decreases and Km increases. Non-competitive inhibition is a special case of mixed inhibition in which the catalytic activity of the enzyme is diminished or abolished, but the ability to bind substrate is unaltered.
Other Mechanisms of Inhibition
While important, especially pharmaceutically, the use of enzyme inhibitors is not the only way to regulate enzymes. There are numerous examples of one type of enzyme activating or inhibiting another. The most common general example are the protein kinases. These enzymes phosphorylate (transfer phosphate group to) other enzymes and thereby activate them. Kinases are generally fast and very specific, and this is an efficient method for activating large numbers of particular enzymes quickly. Conversely, protein phosphatases are enzymes (also quite fast, but much less specific than kinases) that remove the phosphate groups from phosphorylated proteins, thereby turning off those enzymes. Keep in mind that this is a generalization, and that not all phosphorylations are activating. In addition to enzymatic inhibition of enzymes, there is also inhibition by binding and sequestration of the substrates. In fact, the antibiotic vancomycin works just this way, binding to the substrate peptide for transpeptidase and preventing the enzyme from recognizing it. Transpeptidase normally helps stabilize the cell wall of certain bacteria by altering some of the proteins, and without its activity, the protection of the cell wall is compromised and the bacteria may be more easily killed.
Well over half of the enzymes discovered so far do not act in the simplistic Michaelis-Menten one-substrate-one-product mechanism, but rather operate with two substrates and two products, usually with the transfer of an active group. These types of reactions are sometimes known as Bi Bi reactions. There are two major classes of these reactions: the sequential reactions, in which all substrates bind with the enzyme before the reaction proceeds, and the ping pong reactions, in which one or more products are created and released before all of the substrates have been bound. In fact, unlike sequential reactions, the two substrates do not interact with one another while bound to the enzyme.
Optimal Conditions
The activity of enzymes is greatly influenced by both pH and temperature, as expected from the discussion of protein structure in the previous chapter. Activity profiles of most enzymes show a peak of activity that tails off on either side, whether it is pH or temperature. This is an innate characteristic of the enzyme. For example, pepsin, a digestive enzyme secreted into the stomach (pH 2) does not function when the pH > 5. On the other hand, another digestive enzyme, trypsin, which is secreted into the duodenum (proximal small intestine) where the pH is ~8, does not work in acidic environments. Changes in pH can change ionization of amino acid side chains that can thereby alter interaction with the substrate, or lead to changes in tertiary structure.
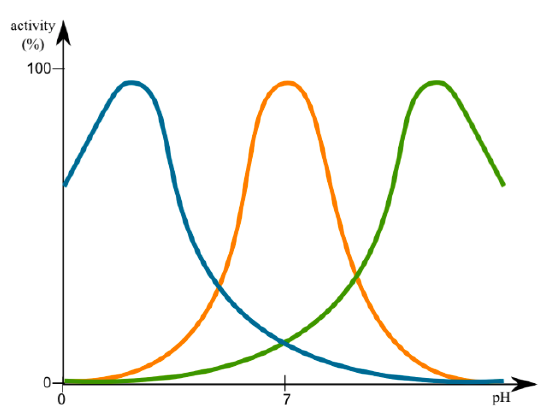
Similarly, at suboptimal temperatures, the likelihood of protein-substrate interaction is low but above the optimal temperature, the increased energy can lead to breaking of hydrogen bonds within the structure of the enzyme, resulting in changes that in- activate the catalytic ability of enzyme or prevent it from binding substrate with sufficient affinity. The temperature optimum of most enzymes is very close to its typical environment. Thus, a human enzyme would operate optimally around 37°C, while an enzyme from bacteria that live in deep-sea volcanic vents (e.g. Thermophilus aquaticus) might have temperature optima over 90°C. This is one of the reasons that refrigeration can slow down growth of microorganisms (which obviously have no ability to regulate their temperature), and why most microorganisms are killed (enzymes permanently denatured) when put into sustained high temperature environments. Interestingly, the DNA polymerase from the T. aquaticus bacteria, also commonly called Taq polymerase, is used in a rapid DNA-amplifying lab technique known as PCR (polymerase chain reaction, see Methods chapter) in which samples are repeatedly heated to high temperatures to separate DNA strands in preparation for making copies of them. DNA polymerases from most prokaryotic or eukaryotic species would be denatured and in- activated by the high heat, but Taq has evolved (with respect to its tertiary structure) for extraordinary structural stability even in heat extremes.
Finally, many enzymes require a molecular partner that has no catalytic activity of its own, but like a catalyst, is not permanently altered by the chemical reaction. These molecules are cofactors. Some are simple: elemental, in fact, including metal ions such as Zn2+ or Ca2+. Others are slightly more complex: small organic cofactors are called coenzymes, and accomplish the same thing, acting as a required partner to the enzyme in catalyzing a reaction. The interaction with the enzyme itself varies and may be only transient, as in NAD+/NADH which are coenzymes used in redox reactions, or permanently bound to the enzyme by covalent bond like the heme group of hemoglobin. Often the function of the coenzyme is to provide an active group to facilitate the catalyzed reaction. Coenzyme A, in various metabolic pathways such as glycolysis or the tricarboxylic acid cycle, can be bound to a substrate to form a stable product that then acts as an intermediate. The Co-A is released from the molecule as it undergoes the next step in a series of reactions in the metabolic pathway (see Chapter 5).
From a human health standpoint, it is interesting to note that many coenzymes are vitamins, or derived from vitamins. These are the B vitamins biotin (B7), cobalamin (B12), folic acid (B3), niacin/nicotinamide (B9), pantothenic acid (B5), pyridoxine (B6), riboflavin (B2), and thiamine (B1). Vitamins are small organic compounds that are not synthesized by an organism and must therefore be ingested. They are generally needed only in small quantities, but necessary nonetheless. Naturally, the vitamins we are familiar with are those required by humans. The specific roles of these vitamins and the con- sequences of not having enough of them are discussed later in this textbook, as the enzymes that they work with are introduced in detail.


