14.3: 7-TM receptors (G-protein-coupled)
- Page ID
- 16179
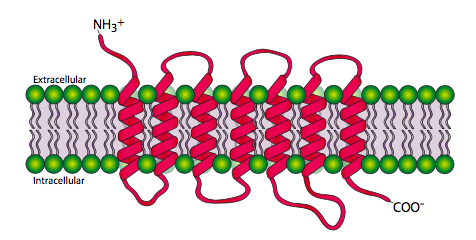
The 7-transmembrane receptors, or G-protein-coupled receptors are, unsurprisingly, a family of proteins that pass through the cell membrane 7 times. The amino terminal is extracellular and the carboxyl terminal is intracellular. Figure \(\PageIndex{2}\) shows the transmembrane regions spread out for clarity, but the transmembrane domains actually form together in more of a cylindrical shape.
7-TM proteins are used as receptors for neurotransmitters such as epinephrine (β-adrenergic receptor), acetylcholine (muscarinic receptor), and serotonin, as well as hormones like glucagon or thyroid-stimulating hormone, and even non-molecular ligands such as light! Rhodopsins are a class of 7-TM receptors that are activated when they absorb a photon (Figure \(\PageIndex{5}\)). Activating this family of receptors, whether by photon or by more conventional ligand binding, induces a conformational change in the cytoplasmic domain that alters the interaction between the receptor and a protein complex known as a heterotrimeric G protein.
The heterotrimeric G protein consists of an a, b, and g subunit, of which the a subunit can bind either GTP or GDP, and can hydrolyze GTP to GDP. When the 7-TM receptor is inactive, the G protein complex is usually nearby associated with the membrane by myristoylation or palmitoylation of the a subunit and farnesylation or geranylgeranylation of the g subunit. Once the 7-TM receptor is activated, it associates with the heterotrimeric G-protein, which causes the Ga to let go of the GDP and bind to a GTP. This then dissociates the Ga from the other two subunits. It can then associate with and activate an enzyme to expand the signaling cascade. One of the two classical path- ways starts with Ga activation of adenylate (adenylyl) cyclase. Adenylate cyclase (AC) converts ATP to cAMP. Since ATP is plentiful and AC is a relatively fast enzyme, the rst ampli cation of the signal comes with generation of the “second messenger” molecule cAMP. Each cAMP molecule can then activate other enzymes, the primary one being protein kinase A. PKA can then phosphorylate a variety of substrates to alter cellular activity by gene expression, molecular motors, or metabolic changes.

The other classical pathway for 7-TM receptors is the activation of phosphlipase Cb, also by Ga. PLCb actually produces two second messenger molecules: it hydrolyzes phosphatidylinositol into diacylgylcerol (DAG) and inositol triphosphate (IP3). IP3 primarily induces the release of Ca2+ from the endoplasmic reticulum. The DAG can activate protein kinase C. PKC is also activated by Ca2+ and both Ca2+ and DAG can activate PKC synergistically. Protein kinase C is an important central kinase that has been shown to phosphorylate and control the activity of numerous other enzymes from cytoskeletal elements to transcription factors.

An interesting variation from the classic 7-TM pathways starts with the rhodopsin receptors in rod cells. These receptors bind photons for activation, and engage a heterotrimeric G protein. The Ga-GTP then binds to the g subunit of phosphodiesterase (PDE), activating it and catalyzing conversion of cGMP to GMP. As cGMP decreases, ion channels close, polarizing the membrane and changing the signal from the rod cell to the brain (via connecting neurons).

Second messengers must have two properties. They must be small enough to diffuse effectively, and the cell must be able to generate them quickly. Ca2+ and cAMP fall into this category. Furthermore they can both be removed from circulation fairly quickly: the former by Ca2+ pumps in the ER and Golgi, and the latter by phosphodiesterase activity. When the G-protein-pathway was discovered, the use of lipid second messengers was surprising. Membrane phospholipids were largely ignored at the time as simple static components of membranes. It is now clear that some of the phospholipids are biochemically active, with several enzymes that modify them, including phospholipases, phospholipid kinases, and phospholipid phosphatases. Some of these enzymes have a variety of functions because their substrate of product may be an important messenger molecule. For example, PI3K (phosphatidylinositol-3-kinase) is a central signaling kinase because its product, PIP3 (phosphatidylinositol (3,4,5)-triphosphate) is an activator for Akt/PKB and other enzymes that can activate several signaling pathways.

The activation must eventually end, and it does so when Ga hydrolyzes the GTP bound to it. In this way, the Ga acts as a kind of timer for the signaling cascade. This is im- portant because for signaling to be effective, it must be tightly controlled. Very early in this course, it was pointed out that Ca2+ is kept at a very low concentration in the cytoplasm of the cell because we want Ca2+-sensitive mechanisms to be able to react quickly to an influx of calcium, but we equally want to be able to quickly turn off the signal as needed, and that is obviously much easier to do by sequestering a small amount of Ca2+ than a lot of it. Similarly, if sustained activity of a recipient cell is called for, it is accomplished by continuous activation of the receptor and not by a long-lasting effect from a single activation. This ensures that if a cellular effect must be abruptly and quickly cut off, it can be accomplished without a significant lag period between cessation of hormone secretion and cessation of intracellular signaling.
The receptor is a part of another shutoff mechanism as well: to prevent overstimulation, the receptors are desensitized for a short time after they have activated. G-protein-coupled receptor kinase (GRK) phos- phorylates the 7-TM receptor. The phosphorylation creates recognition sites for arrestins. The arrestins have a variety of functions, the simplest of which is to act as a competitive inhibitorofG-proteinbindingbythe receptor. This is a relatively short- lived desensitization.

For longer desensitization, arrestin binds to AP2 and clathrin, nucleating formation of a clathrin-coated endocytic vesicle. This removes the receptor from the cell surface, desensitizing the cell for a much longer period of time than simple competition between arrestin and G-protein.
The arrestins have another potential function. They can act as scaffolding proteins that bring a completely different signaling complex to the 7-TM receptor. Figure \(\PageIndex{8}\) shows an example in which the 7-TM receptor is used to activate a Jun transcription factor.
The b-arrestin brings AJK-1, MKKY, and JNK-1 to activate JNK-1, which can then phosphorylate Jun. This allows translocation of phospho-Jun into the nucleus and subsequent dimerization, converting it into an active transcription factor.
What are some of the cellular actions that can be evoked by 7-TM receptor activation? Ca2+ dynamics will be addressed in a separate section. IP3 has been shown to evoke contraction of smooth and skeletal muscle, actin polymerization and cell shape changes, calcium release from intracellular stores, opening of potassium channels, and membrane depolarization. cAMP has been implicated in control of glycogen breakdown and gluconeogenesis, triacyglycerol metabolism, secretion of estrogens by ovarian cells, secretion of glucocorticoids, and increased permeability of kidney cells to water.



