4.1: Membrane Structure and Composition
- Page ID
- 16108
Since most cells live in an aqueous environment and the contents of the cell are also mostly aqueous, it stands to reason that a membrane that separates one side from the other must be hydrophobic to form an effective barrier against accidental leakage of materials or water. In the earlier chapter on the basic biomolecules, cellular membranes were partially defined as being composed primarily of phospholipids: molecules consisting of a phosphorylated polar head group attached to a glycerol backbone that has two long hydrocarbon tails. The composition of the hydrocarbons can vary in length and degree of saturation, and there is also variation in the head groups. It is also important to remember that although we concentrate on the phospholipids as the primary components of the membrane, there are other significant parts: other lipids, including cholesterol, integral and peripheral membrane proteins, and glycosylated lipids and proteins.
Because the phospholipids are amphipathic, meaning they have a hydrophilic head and hydrophobic tail, the simplest conformation for a small group of phospholipids in aqueous solution might be expected to be a micelle (Figure \(\PageIndex{1}\)A), but is this actually the case? Mixtures of hydrophobic molecules and water are thermodynamically unstable, so this structure would protect the hydrophobic fatty acyl tails from the aqueous environment with which the head groups interact. Micelles can form with other amphipathic lipids, the most recognizable being detergents such as SDS (sodium dodecyl sulfate, also called sodium lauryl sulfate), used in common household products such as shampoos. The detergents act by surrounding hydrophobic dirt (1B) and holding it in solution within the micelle to be rinsed away with the water. At smaller sizes, the micelle is fairly stable; however, when there are a large number of phospholipids, the space inside the micelle becomes larger and can trap water in direct contact with the hydrophobic tails (1C). This renders the micelle unstable, so a large single layer of phospholipid is unlikely to serve stably as a biological membrane. Micelles form easily with SDS and other single- tailed lipids because their overall shape (van der Waals envelope) is conical (1D), which lends itself to fitting tight curvatures. However, phospholipids are more cylindrical, and it is harder to t them into a tight spherical micelle. If they do form micelles, they tend to be larger, and likely to collapse.
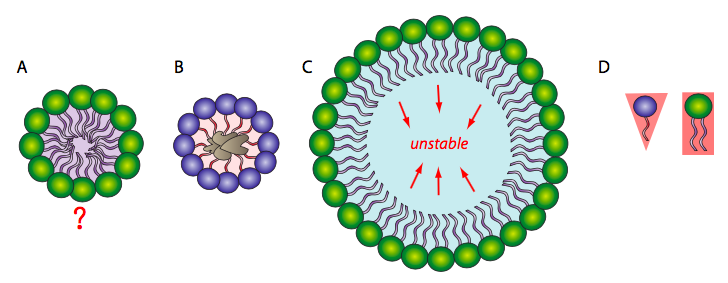
On the other hand, a phospholipid bilayer (Figure \(\PageIndex{2}\)A) could form a fatty acyl sandwich in which the polar head groups face outward to interact with an aqueous environment, and the fatty acids are sequestered in between. However, this does not resolve the problem on the edges of the sandwich. Sometimes, a collapsed micelle can form a closed bilayer in which the edges appear to be sealed, but due to the shape of the phospholipids, there is poor contact between acyl chains. Such a tight bend is unstable and the edge phospholipids are likely to break apart from one another. So, the solution to the ideal phospholipid structure in an aqueous environment is a spherical phospholipid bilayer (2B and cutaway in 2C): no edges mean no exposed hydrophobicity.
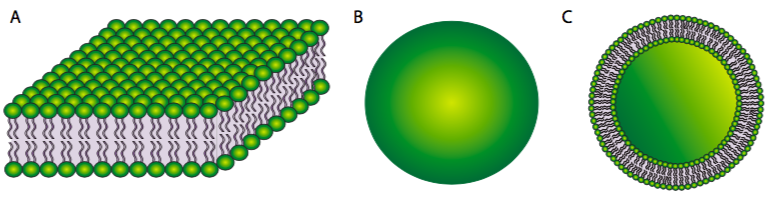
Figure \(\PageIndex{2}\).
The stability of the spherical phospholipid bilayer does not imply that it is static in its physical properties. In most physiologically relevant conditions, the membrane is cohesive, but fluid. It can mold its surface to the contours of whatever it is resting on, and the same thermodynamic and hydrophobic properties that make it so stable also allow it to seal up minor tears spontaneously. Maintaining a working range of fluidity is important to the cell: if the membrane is too rigid, then it may be unable to move or undergo necessary processes such as endocytosis, in which a cell takes up large extra-cellular molecules by enveloping them with the cell membrane and pinching it off in a vesicle; while if it becomes too fluid, it may lose integrity and fall apart.
There are three major factors that govern the fluidity of the membrane:
- degree of saturation of the fatty acyl chains,
- the temperature, and
- the concentration of cholesterol.
Just as overly large micelles will be unstable, there is also a minimal concentration of lipid needed for a micelle to form. This is known as the critical micelle concentration (cmc) and is a property of each particular lipid. Below the cmc, there are not enough amphipathic lipids to mutually shield their hydrophobic tails from the water, and the more likely position of the lipids is on the surface of the aqueous solution, hydrophilic heads in contact, with the hydrophobic tails in the air.
Liposomes are artificial spherical bilayers (Figure \(\PageIndex{2}\)) that are used both in research for study of both membrane lipids and integral membrane proteins. They are also used to deliver drugs and other macromolecules into cells for either research of therapeutic purposes. A common method for getting DNA, a large molecule that is not normally transported into cells, into a cell is called lipofection. This technique involves creating liposomes within a solution containing the DNA of interest. This traps the DNA into the liposome, which can then be applied to cells. The liposomes then fuse with the plasma membrane and deliver the DNA into the cell.
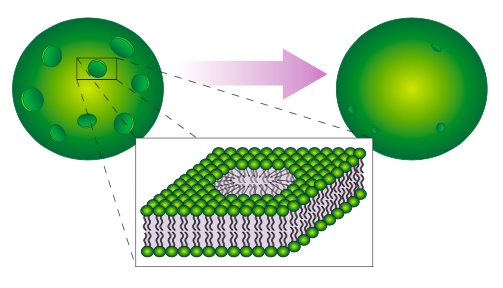
Another technique for introducing foreign DNA into a cell is electroporation, which illustrates the self-sealing property of the phospholipid bilayer (Figure \(\PageIndex{3}\)). The cell (usually thousands of cells, actually) is placed in a DNA solution and subjected to an electric current. This transiently pulls on the cell membrane in all directions, causing many small holes to open up. DNA then moves into the cell, and the holes spontaneously heal. Of course, the process is not perfect, and due to variations in the cells and in the electric field, some cells will be unable to reseal the holes, or for other reasons may not survive the process.
Fully saturated fatty acyl chains can rotate freely around any bond and therefore can pack together very tightly, thus decreasing membrane uidity. As more unsaturated fatty acyl chains are introduced into the membrane, the more space there is between some of the fatty acyl tails, and there is an increase in fluidity. Similarly at higher temperatures, even saturated fatty acyl chains, with their increased energy, move more and create more space between the chains, also increasing fluidity. Finally, cholesterol, as a small planar lipid molecule, can intercalate between the fatty acyl tails. Interestingly, in normal physiological temperatures, the effect of cholesterol is dependent on its concentration. At normal concentrations, the cholesterol restricts acyl tail movement and decreases fluidity. However at very low concentrations, cholesterol has the opposite effect, separating the hydrophobic tails and slightly increasing fluidity, especially in the innermost regions of the membrane. [In case you were wondering, human medical problems with cholesterol are unrelated to the cholesterol in the cell membranes, and refers to cholesterol (bound to lipoprotein carriers) in the bloodstream. That cholesterol can build up in the blood vessels, decreasing the internal diameter and along with it, blood flow. When this happens to the heart, which uses a lot of oxygen, the oxygen deprivation can cause necrosis and a heart attack.]
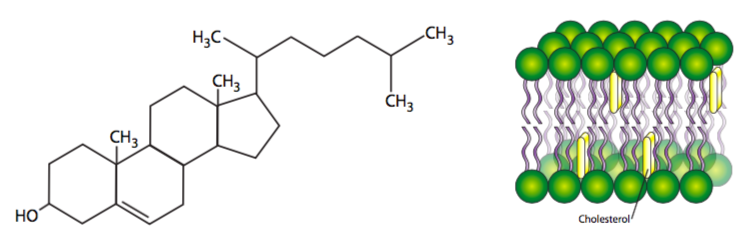
The effect of cholesterol is actually a little more complicated than explained above. Cholesterol is not only planar but rigid, and it intercalates close between acyl tails near the head region. Be- cause cholesterol is somewhat shorter than many acyl tails, while the parts of the hydrocarbon chains nearer the head groups are stabilized and restricted in movement, the cholesterol actually acts as a spacer for the other (methyl) end of each chain, so in the center domain of the hydrophobic core of the bilayer, there is actually increased fluidity around cholesterols. Cholesterol concentration varies greatly among organelles of the same cell, and can even be dynamically regulated in response to temperature changes. Membrane samples taken from fish living in warmer temperatures contain more cholesterol than samples from the same species acclimatized to a lower temperature.
In addition to the three factors noted above, phospholipid composition can also alter membrane fluidity: shorter acyl chains lead to greater fluidity, while longer chains, with more surface area for interaction, generate membranes with higher viscosity. The phospholipid composition of biological membranes is dynamic and can vary widely. The table below shows the differences in the ratios of major phospholipid species phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and sphingomyelin (SM) in plasma membranes from two different cell types. As you might expect based on their differing functions, the ratios of plasma membrane lipids of a myelinating Schwann cell are very different from the lipids in the plasma membrane of a red blood cell.
| Lipid | Schwann Cell PM | Erythrocyte PM |
|---|---|---|
| Phosphatidylcholine | 44% | 19% |
| Phosphatidylethanolamine | 14% | 18% |
| Phosphatidylserine | 3% | 8% |
| Sphingomyelin | 29% | 17% |
Even within a single cell, the composition of the plasma membrane differs from that of intracellular organelles. There is even heterogeneity within a membrane itself - the lipids are not simply distributed randomly in the membrane. Research over the last two decades have identified lipid “rafts” that appear to be speci c for embedding particular proteins. Since they are unanchored lipids, the rafts can move laterally within the membrane just like most individual lipid molecules. Finally, there are different ratios of the lipids between the two layers of the bilayer. The cytoplasmic face of every membrane will have different associations and functions than the non- cytoplasmic face, so why should we expect the lipid composition to be the same?
Though lipid rafts were considered a possibility in the fluid-mosaic membrane model proposed by Singer and Nicholson in 1972, only in the last two decades has the idea been researched seriously, but there have been technical difficulties with visualizing a small domain of lipids within a virtual ocean of lipids. In broad terms, the rafts are considered small areas of ordered lipids within a larger undirected membrane. Lipid rafts most often form in association with specific membrane proteins while excluding others. Some of the proteins are peripheral membrane proteins such as src or the GPI-linked adhesion molecule Thy-1, while others are transmembrane proteins such as the T-cell receptor. Usually the included proteins have signaling-related functions, and one model proposes that these proteins may direct the organization of selected lipids around them, rather than the other way around.
Although some phospholipids are directly linked to proteins and the cytoskeleton, most are not, and are therefore free to move within the plane of its layer of the bilayer. In the experiment shown in Figure \(\PageIndex{6}\) below, the cell surface has been labeled with a covalently bound dye that fluoresces red when excited by green light. When the dye molecules are exposed to high intensity light for an extended period, they no longer fluoresce, a phenomenon known as photobleaching. Photobleaching is a permanent effect, and therefore it can be inferred that if a photobleached spot fluoresces again, then other, non-bleached, phospholipids must have moved into the spot. This experiment clearly proves the lateral mobility of phospholipids in a membrane.
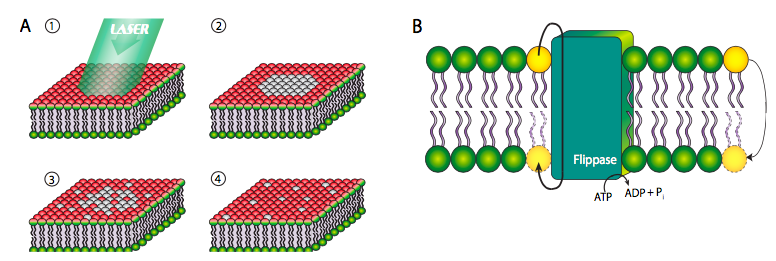
However, this is not the case with transverse mobility from one face of the membrane to the other. Due to the highly unfavorable energetics of pushing a polar head group through the lipid tail layers, phospholipids very rarely move from one layer to the other within a membrane (Figure \(\PageIndex{6}\)B, far right yellow phospholipid). Such movement is greatly facilitated by a ippase enzyme, which hydrolyzes a molecule of ATP for the energy to push a phospholipid from one face of the bilayer to the other.
In a pure phospholipid bilayer, membrane proteins as well as lipids have lateral mobility, but in living cells, the proteins are generally constrained either by preferential association with certain kinds of lipids, by direct attachment to cytoskeletal elements, by in- direct attachment to the cytoskeleton, or by being “fenced in” by cytoskeletal gridwork directly underlying the cell membrane. Since 1972, the Singer-Nicholson “fluid mosaic” model of membrane structure has been accepted as a general model for biological membranes. It proposes that integral membrane proteins as well as membrane lipids have lateral freedom of movement. This has since been re ned with the recognition of lipid rafts and clustered membrane protein patches, but is still viable as a basic model.
For over a century since Meyer (1899) and Overton (1901) first suggested it, the prevailing theory for the mechanism of gaseous general anesthesia (e.g. ethyl ether, halothane, nitrous oxide, cyclopropane) has been that they partition into and interact with the lipids of the plasma membranes of neurons and by altering the physical membrane properties accomplish the anesthesia. However, in 1985, Franks and Lieb published a report in Nature that, for the first time, showed that an enzyme could be directly affected by a gaseous anesthetic. Since that report, the evidence has been building against the old model of altering membrane lipid properties, and now the current model of direct gas-protein interaction is assumed.


