6.3: The Behavior of Water
- Page ID
- 29544
Because all atoms are constantly in motion and because this motion is random, atoms tend to disperse relatively evenly across an environment because this arrangement has a higher probability of occurring. This process is called diffusion. When you drop food coloring into a glass of water, the molecules in the food coloring bounce off of each other and begin to gradually disperse away from each other. The water molecules do the same until the molecules of food coloring and the molecules of water are relatively evenly dispersed throughout the glass. The molecules don’t stop moving, but due to the random motion, the mixture appears to remain evenly mixed.
Osmosis
Osmosis is the diffusion of water across a semipermeable membrane. Because the membrane is semipermeable, water can move through the membrane, but other dissolved compounds may not be able to, for example dissolved ions like Na+ and Cl-. This has some interesting implications.
Tonicity
To think about osmosis, it is important to consider the concentration of water molecules relative to the amount of stuff dissolved in it on either side of the membrane. This concept is called tonicity -- how much stuff is dissolved in the solution relative to the other side.
Water will move from areas of high water concentration to areas of low water concentration. Even if you have the same volume of water on both sides of a membrane, the water will move to the side of the membrane with more dissolved solutes, which can also be interpreted as relatively less water.
Hypotonic
How does this influence plant structure and function? Plants use tonicity to provide increased structural support by keeping plant cells in a turgid state (swollen with water). Plants sequester dissolved solutes in the central vacuole, making the solution hypotonic.
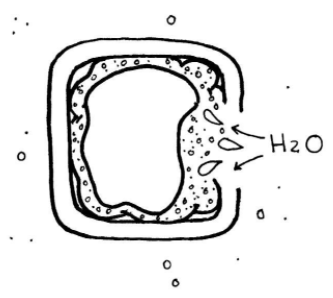
Considering your knowledge of root words, what do you think the term hypotonic means?
Because the concentration of water is relatively low inside the central vacuole (due to the high concentration of solutes), this causes water to move from the exterior environment, where it is at a higher concentration, into the central vacuole. The cell wall keeps the plasma membrane from exploding as the central vacuole swells with water and pushes the cytoplasm outward. Think of it like blowing up a balloon inside a cardboard box--the tension of the inflated balloon provides structural support to the box. The cell above is in a hypotonic solution.
Isotonic
For animal cells, which lack a cell wall, being in a hypotonic solution causes cells to swell and burst. Our cells remain in an isotonic solution (iso- meaning equal or identical), with the concentration of dissolved solutes (and therefore water) approximately the same in the intracellular and extracellular fluids. The cell in the diagram is in an isotonic solution. Plant cells in this state are slightly wilted.

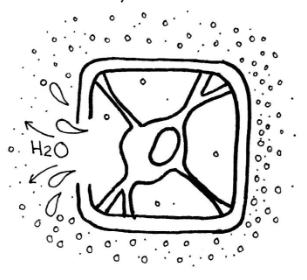
Hypertonic
The third possibility is that the concentration of solutes is higher in the solution than inside the cell, a hypertonic solution (hyper- meaning over or above). This means that the concentration of water is relatively higher inside the cell than outside, causing the water to move out. This is why salt can dehydrate (pull water out of) things, including organisms. If a plant that is not adapted to saltwater is submerged in a solution of saltwater, or perhaps there is too much salt in the soil, water will exit the plant cells to enter the solution. The cell in the diagram on the right is in a hypertonic solution.
To observe the effects of tonicity on plant cells, prepare a wet mount of an Elodea leaf (or similar freshwater plant). For the initial preparation, use pond water or water from the tank the Elodea was growing in. Observe at 400x magnification.
Draw a few cells in the space below, labeling the cell wall, plasma membrane, chloroplasts and tonoplast. The tonoplast is the membrane of the central vacuole. Though you cannot see it directly, you can infer the location of the tonoplast by where the chloroplasts are--they line the outside of it.
Remove the coverslip, soak up as much of the water as you can, and add a few drops of saltwater (sodium chloride, NaCl, solution) to your slide. Quickly place back under the microscope and view at 400x. Once you see a change in the cells, draw a few of them. Label the cell wall, plasma membrane, chloroplasts and tonoplast.
What happened to the cell? Was the solution hypotonic, isotonic, or hypertonic?
In your drawing, use arrows to depict the movement of water either entering or exiting the central vacuole.
Soak up as much of the saltwater as you can, and this time add a few drops of distilled water (water that has had the dissolved solutes removed) to your slide. Quickly place back under the microscope and view at 400x. Once you see a change in the cells, draw a few of them. Again, label the cell wall, plasma membrane, chloroplasts and tonoplast.
What happened to the cell? Was the solution hypotonic, isotonic, or hypertonic? Explain the movement of the water molecules in your answer.


