4.3.1: Essential Elements
- Page ID
- 32005
Learning Objectives
- List the essential elements required by plants and summarize their functions.
- Describe how plants obtain nutrients, including the mechanism of cation exchange.
- Distinguish between macronutrients and micronutrients.
Plants are unique organisms that can absorb nutrients and water through their root system, as well as carbon dioxide from the atmosphere. Soil quality and climate are the major determinants of plant distribution and growth. The combination of soil nutrients, water, and carbon dioxide, along with sunlight, allows plants to grow.
The chemical composition of plants reflects the essential elements, which are necessary for plant growth and reproduction. For an element to be regarded as essential, three criteria are required: 1) a plant cannot complete its life cycle without the element; 2) no other element can perform the function of the element; and 3) the element is directly involved in plant nutrition. There is some disagreement of the number of essential elements for plants with experts listing as few as 15 or as many as 20. Nineteen essential elements are discussed here. While identifying essential elements may seem straightforward, the nutritional needs of plants somewhat depend on the species and environmental conditions. As a result, some may argue that certain elements, like cobalt (Co), are essential, but it is typically only considered a beneficial element.
Chemical Composition of Plants
The majority of the plant body consists of carbon (C), hydrogen (H), and oxygen (O). Plants obtain carbon from carbon dioxide in the atmosphere and hydrogen from the water absorbed by the roots. Oxygen atoms come from carbon dioxide and gaseous oxygen in the atmosphere, as well as from water. Water typically comprises 80 to 90 percent of the plant’s total weight. However, carbon and oxygen constitute about 45% of dry plant tissue (biomass) each, and hydrogen makes up 6%. The remaining 4% of dry biomass consists of elements that are obtained from the soil. These mineral nutrients are categorized as macronutrients and micronutrients.
Macronutrients are required in relatively large quantities (more than 0.1% of dry biomass). The macronutrients in order of contribution to dry biomass are nitrogen (N), potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), sulfur (S), and silicon (Si). Nitrogen, potassium, and phosphorus are major components in fertilizer (figure \(\PageIndex{1}\)). Silicon is only absolutely required by horsetails, but many other plant species contain silicon and benefit from its presence. Some sources consider carbon, oxygen, and hydrogen macronutrients. However, this text will not because they are obtained from the atmosphere and/or water rather than minerals in the soil.


Micronutrients are required in small quantities (less than 0.01% of dry biomass). Plant micronutrients are chlorine (Cl), iron (Fe), boron (B), manganese (Mn), sodium (Na), zinc (Zn), copper (Cu), nickel (Ni), and molybdenum (Mo). Sodium is primarily required by plants using certain photosynthetic pathways (C4 and CAM), but like silicon, it benefits many plant species.
Absorption of Mineral Nutrients
Plants absorb most mineral nutrients from the soil as ions. Some of these essential elements are cations, including potassium (K+), calcium (Ca2+), magnesium (Mg2+), iron (Fe3+ or Fe2+), manganese (Mn2+), sodium (Na+), zinc (Zn2+), copper (Cu+ and Cu2+), and nickel (Ni2+). Other nutrients are found in the form of anions, including dihydrogen phosphate (H2PO4-) or hydrogen phosphate (HPO42-), sulfate (SO42-), chloride (Cl-), and molybdate (MoO42-). Plants obtain nitrogen from the soil as nitrate (NO3-) or ammonium (NH4+). Boron is absorbed as boric acid (H3BO3) or its conjugate base, dihydrogenborate (H2BO3-). Silicon is available as silicic acid (H4SiO4).
Cations in the soil are bound to negatively charged clay particles or the organic acids that form humus (see Soils), and this makes it difficult for plants to absorb them. Plants have a mechanism called cation exchange, which releases cations and frees them for absorption (figure \(\PageIndex{2}\)). This occurs when the roots pump protons (H+) into the soil. The protons bind to the clay and humus, taking the place of the cation nutrients, such as K+ ,Ca2+, and Mg2+. These nutrients are then freely dissolved in the water in the soil and can enter the roots. Roots can also increase proton concentration (decrease pH) of the soil indirectly by releasing carbon dioxide, which reacts with water to form carbonic acid. Protons released when carbonic acid molecules disassociate can then contribute to cation exchange.
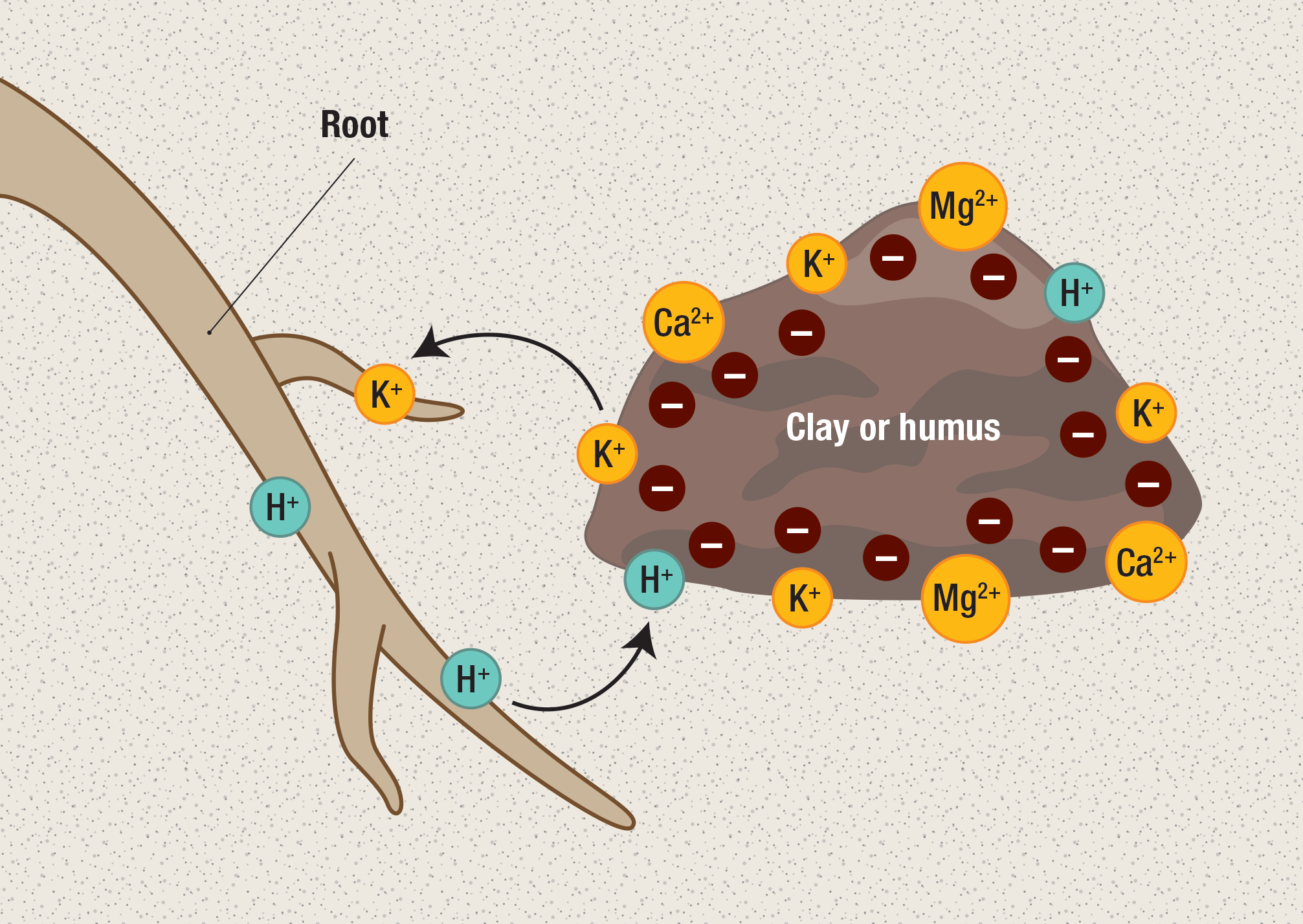
To absorb iron, plants must either release protons creating acidic conditions, which promote the conversion (reduction) of Fe3+ to Fe2+, or produce special compounds called siderophores. These bind to Fe3+ forming a complex, which can then be transported into the root.
Because anions are not attracted to clay and humus in the soil, it is easier for them to leach from the soil when it is irrigated or when it rains. For this reason, anions like nitrates and phosphates are common causes of eutrophication (see Threats to Biodiversity).
Mycorrhizae, the symbiotic fungi that grow around or inside of root cells, help plants absorb a variety of mineral nutrients, particularly phosphorus.
Functions of Essential Elements
Each essential element has multiple roles. For example, calcium acts a second messenger, transmitting signals within a cell, and as a cofactor, assisting enzyme function. While multiple functions for most of the essential elements are described below, they are organized based on one of their primary functions.
Elements in Biological Macromolecules
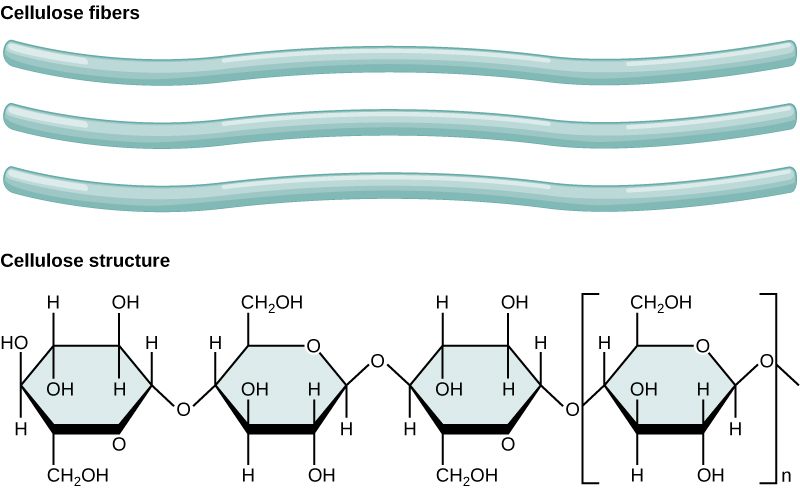
Carbon dioxide is a reactant in photosynthesis, and carbon is required to form biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids). For example, the carbohydrate cellulose is the main structural component of the plant cell wall and makes up over thirty percent of plant matter (figure \(\PageIndex{3}\)). Besides the biological macromolecules, plants contain many other organic molecules, such as the chlorophyll and plastoquinone, and all of these contain carbon. In fact, organic molecules are defined as those that contain carbon and hydrogen, typically have carbon-to-carbon bonds, and are often larger and more complex than inorganic molecules.
Hydrogen and oxygen are components of water and all of the biological macromolecules. Hydrogen is found in all organic compounds, and oxygen is found in many, as well. Gaseous oxygen is also a reactant in aerobic cellular respiration.
Nitrogen is part of proteins, nucleic acids, and chlorophyll. Nitrogen is also used in the synthesis of some vitamins, such as vitamin B6, which serves as a coenzyme in protein synthesis.
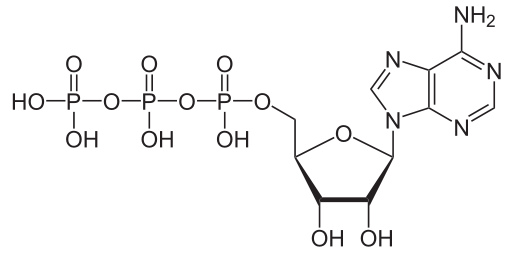
Phosphorus is necessary to synthesize nucleic acids and phospholipids, which form the plasma membrane, chloroplasts, and many other plant cell structures. Adenosine triphosphate (ATP), the primary form of ready-to-use energy in the cell, contains three phosphate groups, each with a phosphate in the center (figure \(\PageIndex{4}\)). Plants generate ATP by binding a phosphate group with adenosine diphosphate (ADP) through oxidative phosphorylation during cellular respiration and during photophosphorylation in photosynthesis.
Sulfur is part of certain amino acids, such as cysteine and methionine, and is present in several coenzymes. For example, it connects coenzyme A to acetyl groups, forming acetyl CoA, the starting molecule for the Krebs cycle (tricarboxylic acid cycle) of cellular respiration. Sulfur also plays a role in photosynthesis as a component of the iron sulfur proteins that transfer electrons from photosystem I to NADP+.
Elements that Maintain Ion Balance
Potassium and chlorine play roles in regulating stomatal opening and closure. As the openings for gas exchange, stomata help maintain a healthy water balance. This process is supported by a potassium ion pump as well as the movement of chloride through symport and anion channels. Potassium is also a cofactor for many enzymes, including those involved in photosynthesis. Chlorine, along with calcium, is essential for water photolysis, which generates oxygen during photosynthesis. Calcium also transmits signals within the cell (including as a second messenger during stomatal closure), acts as a cofactor for many enzymes, and contributes to cell wall structure at the middle lamellae between adjacent cells.
Elements Involved in Enzyme Function
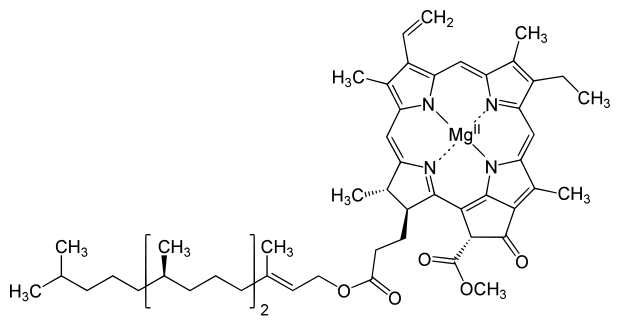
Magnesium, zinc, nickel, copper, and manganese all function as cofactors. Specifically, manganese assists with water photolysis. Zinc aids with the synthesis of chlorophyll. Nickel is involved breaking down urea. Copper can serve as a cofactor or a component of the enzyme itself. For example, the copper in plastocyanin allows it to transport electrons during photophosphorylation. In addition to aiding with enzyme function, magnesium is additionally important to the photosynthetic process because it is part of chlorophyll (figure \(\PageIndex{5}\)).
Like copper, iron facilitates electron transport in enzymes. It is found in the cytochromes involved in oxidative phosphorylation and photophosphorylation. Iron is also plays a role in synthesizing chlorophyll. Molybdenum is a component of a few enzymes in plants, including one that helps plants use nitrate.
Sodium helps some plants synthesize phosphoenolpyruvate (PEP). Carbon dioxide is added to PEP to form oxaloacetate during C4 and CAM photosynthesis.
Elements in Cell Walls
Of course, polysaccharides such as cellulose, which contain carbon, hydrogen, and oxygen, are the main components of plant cell walls. However, other elements play more minor roles. Calcium in the middle lamella was already mentioned. Additionally, silicon plays a role in cell wall structure in horsetails. Boron is involved in cell wall structure and elongation.
Nutrient Deficiencies
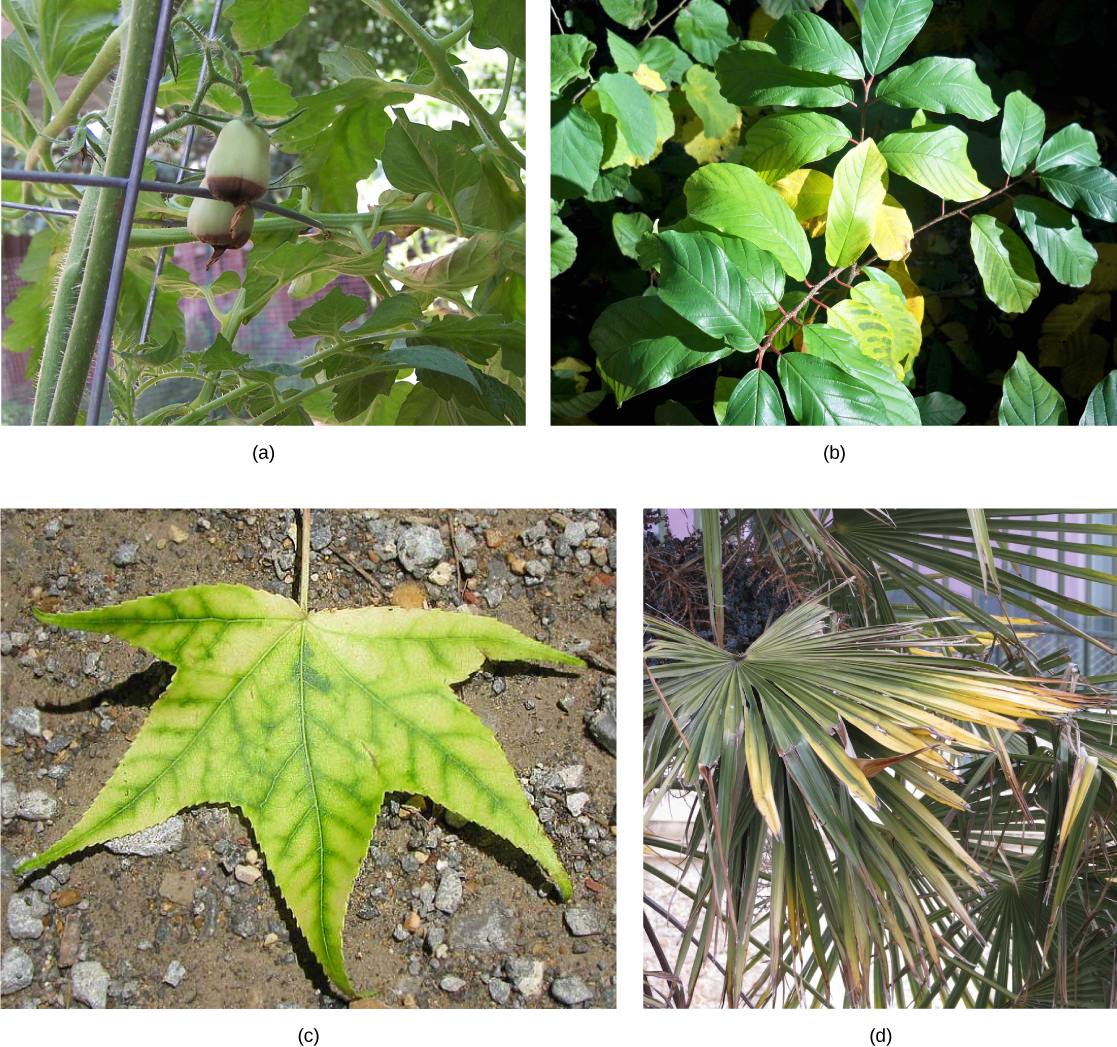
Deficiencies in any of these nutrients—particularly the macronutrients—can adversely affect plant growth (Figure \(\PageIndex{6}\)). Depending on the specific nutrient, a lack can cause stunted growth, slow growth, or chlorosis (yellowing of the leaves). Extreme deficiencies may result in leaves showing signs of necrosis (death of the tissues). The location of the symptoms, such as in old leaves versus young leaves, can also be telling. Some nutrients like iron are immobile, so an iron deficiency would cause chlorosis in young leaves. On the other hand, magnesium can be transported from old leaves to developing ones. As a result, a magnesium deficiency would be manifested as chlorsis in the old leaves.
Everyday Connection: Hydroponics
Hydroponics is a method of growing plants in a water-nutrient solution instead of soil (Figure \(\PageIndex{7}\)). Since its advent, hydroponics has developed into a growing process that researchers often use. Scientists who are interested in studying plant nutrient deficiencies can use hydroponics to study the effects of different nutrient combinations under strictly controlled conditions. Hydroponics has also developed as a way to grow flowers, vegetables, and other crops in greenhouse environments. You might find hydroponically grown produce at your local grocery store. Today, many lettuces and tomatoes in your market have been hydroponically grown.

Attribution
Curated and authored by Melissa Ha using 31.1 Nutritional Requirements of Plants from Biology 2e by OpenStax (CC-BY). Access for free at openstax.org.


