4.1.1: Energy and ATP
- Page ID
- 50920
Learning Objectives
- Describe the different types of energy.
- Describe the structure and function of ATP.
Understanding photosynthesis and aerobic cellular respiration relies on the fundamentals of energy. Energy is defined as the ability to do work, and there are several types of energy (Figure \(\PageIndex{1}\)). Kinetic energy is the energy of motion. Examples include a ball rolling down a hill, heat energy, and light energy. Heat energy is technically energy that is transferred between systems without doing work. The higher the temperature, the faster molecules in matter move. Potential energy is the energy that matter possesses but is not currently being used. For example, a ball sitting a the top of the hill that has not yet rolled down the hill possesses potential energy. Chemical energy is an example of potential energy that is stored in molecules. When molecules that are higher energy and less stable react to form products that are lower energy and more stable, this stored energy is released.

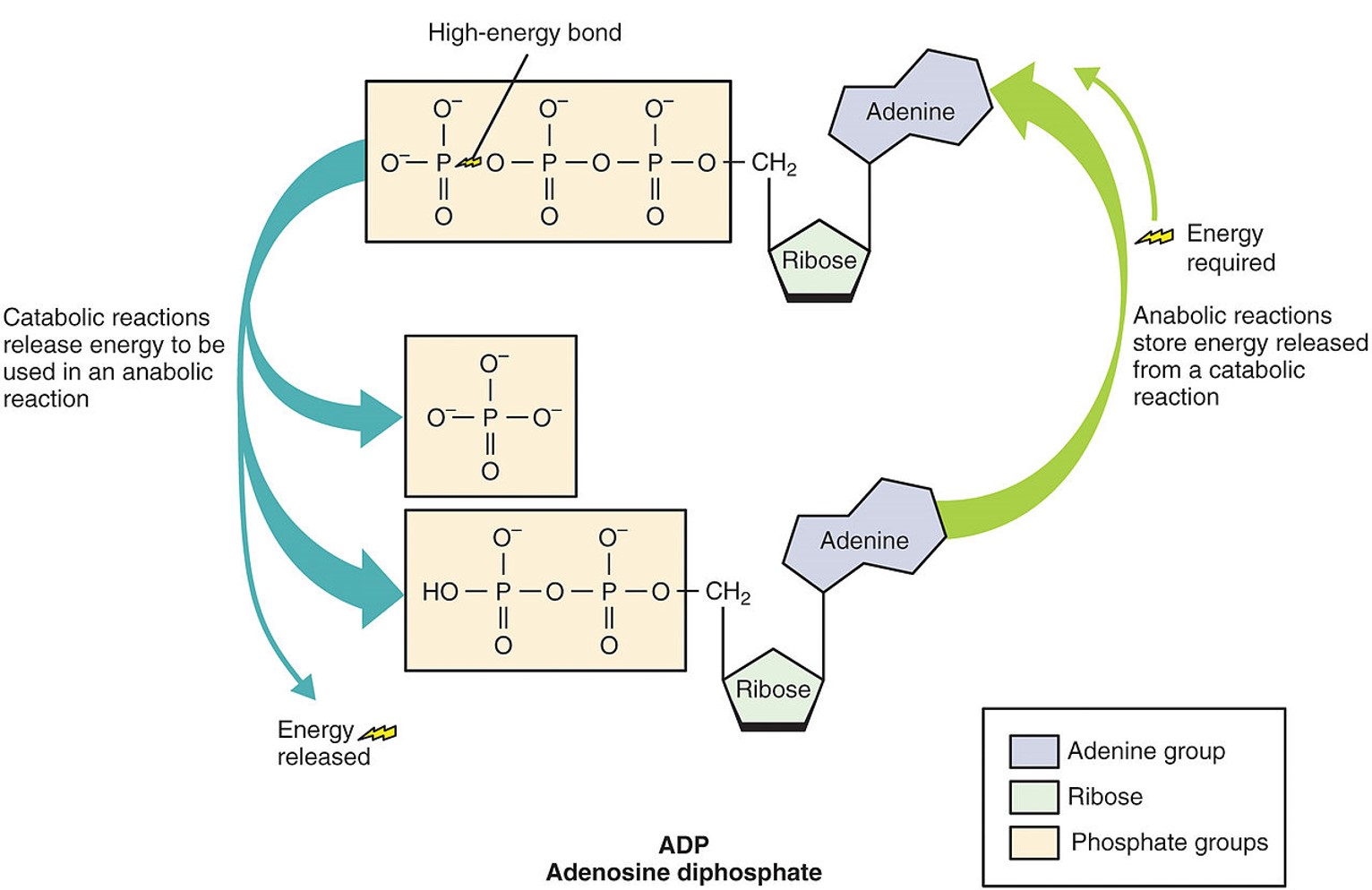
Adenosine triphosphate (ATP) is considered the energy currency of the cell because it provides usable energy. Structurally, ATP resembles a modified nucleotide (the building blocks of DNA and RNA). Specifically, it consists of adenine, ribose, and three phosphate groups (Figure \(\PageIndex{2}\)). The bonds between the phosphate groups are unstable. When these bonds are broken, more stable bonds are formed in their place, releasing energy. Phosphorylation refers to adding a phosphate group (PO43-) to a molecule. However, it often refers specifically to synthesizing ATP by adding a phosphate group to adenosine diphosphate (ADP).