1.2: Styles of Life and Basic Chemistry
- Page ID
- 17977
Life obtains energy in a few different ways: (1) from sunlight (phototrophy); (2) from chemical reactions with inorganic matter (lithotrophy); (3) from breaking organic molecules into inorganic molecules, typically carbon dioxide and water (organotrophy). To make its body, living beings obtain building blocks either by (a) from the assimilation of carbon dioxide (autotrophy), or from other living beings (heterotrophy).
These ways combine in six lifestyles. For example, plants\(_1\) are by definition photoautotrophs. Most plants\(_2\) are also photoautotrophs, but there are exceptions: full parasites (see above). Carnivorous plants (like sundew, Drosera or the Venus flycatcher, Dionaea) are all photoautotrophs. They “eat” animals in order to obtain nitrogen and phosphorus, so the dead bodies serve not as food but as a fertilizer. Note that plants are also organoheterotrophs like animals because in addition to photosynthesis, all plant cells can respire.
To understand life of plants, a basic knowledge of chemistry is needed. This includes knowledge of atoms (and its components like protons, neutrons and electrons), atomic weight, isotopes, elements, the periodic table, chemical bonds (ionic, covalent, and hydrogen), valence, molecules, and molecular weight. For example, it is essential to know that protons have a charge of \(+1\), neutrons have no charge, and electrons have a charge of \(-1\). The atomic weight is equal to the weight of protons and neutrons. Isotopes have the same number of protons but different number of neutrons; some isotopes are unstable (radioactive).
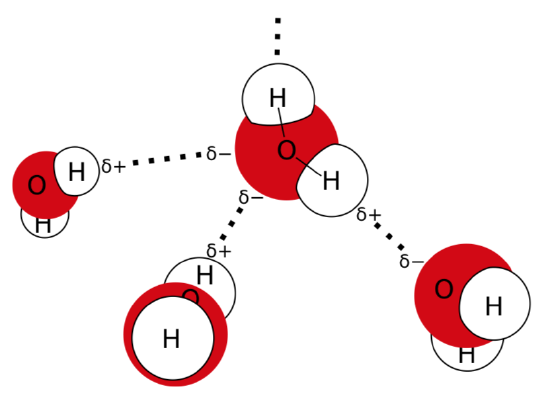
One of the most outstanding molecules is water. Theoretically, water should boil at much lower temperature, but it boils at 100\(^\circ\)C just because of the hydrogen bonds sealing water molecules. These bonds arise because a water molecule is polar: hydrogens are slightly positively charged, and oxygen is slightly negatively charged (Figure \(\PageIndex{1}\)).
Another important concept related to water is acidity. If in a solution of water, the molecule takes out proton (H\(^+\)), it is an acid. One example of this would be hydrochloric acid (HCl) which dissociates into H\(^+\) and Cl\(^-\). If the molecule takes out OH\(^-\) (hydroxide ion), this is a base.
An example of this would be sodium hydroxide (NaOH) which dissociates into Na\(^+\) and hydroxide ion.
To plan chemical reactions properly, we need to know about molar mass and molar concentration. Molar mass is a gram equivalent of molecular weight. This means that (for example) the molecular weight of salt (NaCl) could be estimated as \(23 + 35\), which equals 58 units. Consequently, one mole of salt is approximately 58 grams. One mole of any matter (of molecular structure) always contains \(6.02214078 \times 10^{23}\) molecules (Avogadro’s number).
The density of a dissolved substance is the concentration. If in 1 liter of distilled water, 58 grams of salt are diluted, we have 1M (one molar) concentration of salt. Concentration will not change if we take any amount of this liquid (spoon, drop, or half liter).
Depending on the concentration of protons in a substance, a solution can be very acidic. The acidity of a solution can be determined via pH. For example, if the concentration of protons is 0.1 M (\(1 \times 10^{-1}\), which 0.1 grams of protons in 1 liter of water), this is an extremely acidic solution. The pH of it is just 1 (the negative logarithm, or negative degree of ten of protons concentration). Another example is distilled water. The concentration of protons there equals \(1 \times 10^{-7}\) M, and therefore pH of distilled water is 7. Distilled water is much less acidic because water molecules dissociate rarely.
When two or more carbon atoms are connected, they form a carbon skeleton. All organic molecules are made of some organic skeleton. Apart from C, elements participate in organic molecules (biogenic elements) are H, O, N, P, and S. These six elements make four types of biomolecules: (1) lipids—hydrophobic organic molecules which do not easily dissolve in water; (2) carbohydrates or sugars, such as glucose (raisins contain lots of glucose) and fructose (honey); by definition, carbohydrates have multiple \(-\)OH group, there are also polymeric carbohydrates (polysaccharides) like cellulose and starch; (3) amino acids (components of proteins) which always contain N, C, O and H; and (4) nucleotides combined from carbon cycle with nitrogen (heterocycle), sugar, and phosphoric acid; polymeric nucleotides are nucleic acids such as DNA and RNA.


