13.3: Cellular Respiration
- Page ID
- 29572
Step 1: Glycolysis
When glucose is transported into the cytoplasm of cells, it is broken down into two molecules of pyruvate. This process is called glycolysis (glyco- for glucose and -lysis, meaning to break apart). Glycolysis involves the coordinated action of many different enzymes. As these enzymes start to break the glucose molecule apart, an initial input of energy is required. This initial energy is donated by molecules of ATP.
Though two molecules of ATP are used to get glycolysis going, four more molecules of ATP are produced during the reaction, resulting in the net production of two ATP per molecule of glucose. In addition to ATP, two molecules of NAD+ are reduced to form NADH. When a molecule is reduced, electrons have been added to it. Electrons have a negative charge, so this is termed “reduction”. When NAD+ is reduced to NADH, two high energy electrons derived from breaking the bonds of glucose are added to it. One of those negatively charged electrons is balanced by the positive charge (+) on NAD+. The other is balanced by adding a proton (\(\ce{H+}\)) to the molecule. Because NADH carries two high energy electrons, it is often referred to as an electron carrier.
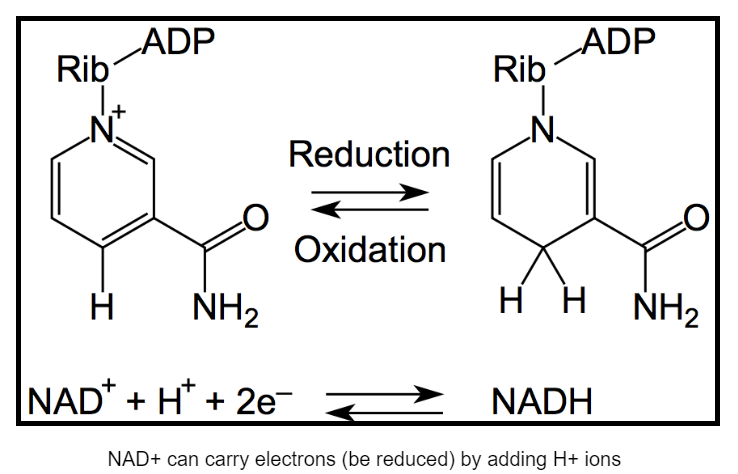
|
Reactants (What went in?) |
Products (What came out?) |
|---|---|
|
1 Glucose |
|
|
2 NAD+ |
|
|
2 ADP (net) |
Alternate Pathways: Fermentation
At this point, cells make a check: Is there oxygen present or not? If not, some organisms can go through a process called fermentation. In fermentation, glycolysis is the only part of glucose breakdown that a cell can do. Thus, only two net ATP can be gained from each glucose molecule. To continue doing fermentation, the cell must regenerate the NAD+ needed to do glycolysis.
There are two primary pathways to regenerate NAD+. In your body, cells can regenerate NAD+ by producing lactate and \(\ce{H+}\). This is called lactic acid fermentation, though lactic acid is never actually produced, so it is a bit of a misnomer. Some bacteria, like Lactobacillus, are also capable of doing this type of fermentation.
Making Yogurt With Lactic Acid Fermentation
Put some milk into a flask and add a spoonful of yogurt. Mix thoroughly, cover, and place in a warm environment. Over the next 24 hours, the sugars in the milk will be fermented into lactate and H+ ions. This thickens the milk and adds acidity, making yogurt.
Why did you add the spoonful of yogurt to start it? What did this add to the milk that would allow fermentation to occur?
Why did you need to put it into a warm environment?
Do you think the type of milk you use affects the time it takes to get yogurt? Explain your answer.
Another pathway to regenerate NAD+ is to produce \(\ce{CO2}\) and ethanol (alcohol). Certain yeasts, like Saccharomyces cerevisea, perform this type of alcohol fermentation when they do not have access to oxygen.
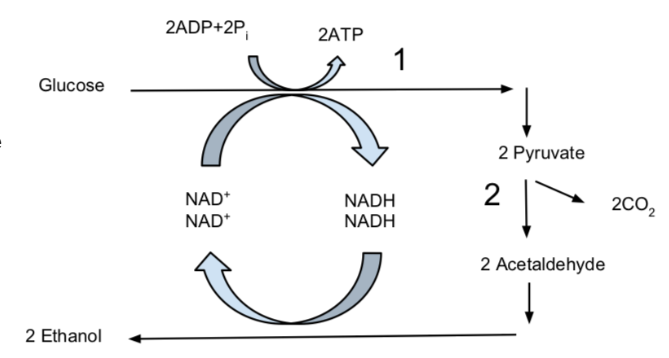
Experimental Design: Rates of Alcoholic Fermentation
For this experiment, you will be attempting to answer the following question:
“What factors influence the rate of fermentation by yeast?”
You will have the choice between one of two independent variables. The independent variable is the variable you are asking questions about. It should be the only thing that you establish as different between your treatment groups:
- Type of juice used
- Temperature of fermentation chamber
Why would it be important to only have one independent variable?
For the dependent variable, you will measure the circumference of a balloon that has been placed over your fermentation flask. The dependent variable is the one that you measure to determine the influence of the independent variable on your different treatments.
Why would the circumference of the balloon be a good indicator for the rate of fermentation? What will the balloon be collecting?
Choose an independent variable and make a prediction on how it will influence the dependent variable. This is called your hypothesis.
To measure the effects of the independent variable on the rate of fermentation, you’ll need to establish treatment groups and controls. Your treatment groups should have some modification to the independent variable (such as different temperatures or different types of juice). Choose three different variations of your independent variable and record them below:
T1:
T2:
T3:
Controls are established to account for any additional variables within the experiment, as well as to give you information on whether your experiment actually worked. A positive control should always work (give expected results). In this experiment, fermentation should definitely occur in the positive control for this experiment. If fermentation does not occur in the positive control, you know something about your experiment is off and you could be getting false negatives. Think of a positive control you could use for your experiment and record it below:
Positive control (C+):
A negative control is just the opposite. In a negative control, you want to see no change from initial conditions. If there is a change in the dependent variable in the negative control, this gives you a baseline of variability that you can expect in the absence of changes to the independent variable. For example, in this experiment, a negative control should have no fermentation occurring and thus, no inflation of the balloon. However, gases can expand in higher temperatures, so a negative control placed in a warmer environment would have expansion of the balloon. This gives us some baseline data for how temperature will affect the gases in the balloon without the influence of fermentation. Think of a negative control you could use for your experiment and record it below (you may need more than one):
Negative control(s) (C-):
Now that you have designed the experiment, it is time to run it and collect data on the dependent variable. Enter your results into the table below:
|
Treatment Group |
Initial Circumference (cm) |
Final Circumference (cm) |
Change in Circumference (cm) |
|---|---|---|---|
|
Control |
Initial Circumference (cm) |
Final Circumference (cm) |
Change in Circumference (cm) |
|---|---|---|---|
|
C+ |
|||
|
C- |
Once you have collected your data, you need to interpret it and draw conclusions about your hypothesis.
Does the data support your hypothesis or not? Explain your answer.
Were there any variables that could have influenced your experiment that you did not control for? If so, how could you adjust this experiment to account for these on a future trial?
Consult as a class about your findings. Did everyone perform the experiment the same? Did they get the same results?
Compare the results of the class for the two different independent variables. Which had a stronger influence on rate of fermentation: the type of juice or the temperature? Attempt an explanation for these findings.
Connecting back to cellular respiration, how much ATP is produced from one molecule of glucose if a cell only goes through glycolysis?
Why do organisms ferment instead of going through the process of cellular respiration?
We used the balloon to collect gases produced during fermentation. However, the balloon was necessary for another reason (hint: it relates to the previous question). Why else did we cover the flask with the balloon?
Step 2: The Link Reaction
If oxygen is present, cellular respiration can continue (more on this in the next section). The two molecules of pyruvate are transported into the matrix of the mitochondrion. During transport, each pyruvate is converted into a 2-carbon molecule called acetyl-\(\ce{CoA}\). The other carbon atom from each pyruvate molecule exits the cell as \(\ce{CO2}\). The electrons from this broken bond are captured by another molecule of NAD+, reducing it to NADH.
Step 3: The Citric Acid (Krebs) Cycle
The acetyl-\(\ce{CoA}\) enters a cycle which, much like glycolysis, involves the action of many different enzymes to release energy and transport it in energy-carrying molecules: ATP, NADH, and another electron carrier, \(\ce{FADH2}\). This cycle takes place within the matrix of the mitochondrion.
In the space below, draw a mitochondrion in a cell. Show where glycolysis, the link reaction, and citric acid cycle happen. It may help you to include the compounds involved in each stage, such as glucose, NAD+, \(\ce{CO2}\), and others.
Step 4: Oxidative Phosphorylation
This stage of cellular respiration has two steps. During the electron transport chain, our electron carriers power a series of proton pumps that move \(\ce{H+}\) ions from the mitochondrial matrix to the space between the inner and outer mitochondrial membranes. During chemiosmosis, an enzyme called ATP synthase allows the protons to flow back into the mitochondrial matrix, using the physical flow of the protons to turn ADP into ATP.
The Electron Transport Chain
NADH and \(\ce{FADH2}\) drop off their electrons at a protein complex within the inner mitochondrial membrane. This effectively “turns on” this protein complex, which pumps a \(\ce{H+}\) from the mitochondrial matrix to the intermembrane space. The electrons are then passed down a line of protein complexes, much like a current of electricity, powering these complexes to each pump a \(\ce{H+}\) from the matrix into the intermembrane space. This is appropriately named the electron transport chain.
At the end of the electron transport chain, the low energy electrons need to be picked up to make space for more electrons. An oxygen atom picks up two electrons and, to balance the charge, two \(\ce{H+}\) from the matrix, forming a water molecule (\(\ce{H2O}\)). In cellular respiration, oxygen is the terminal electron acceptor, because it picks up the electrons at the end (the terminus) of the electron transport chain. This job is so important that, as you saw above, if oxygen is not present, this part of cellular respiration will not occur.
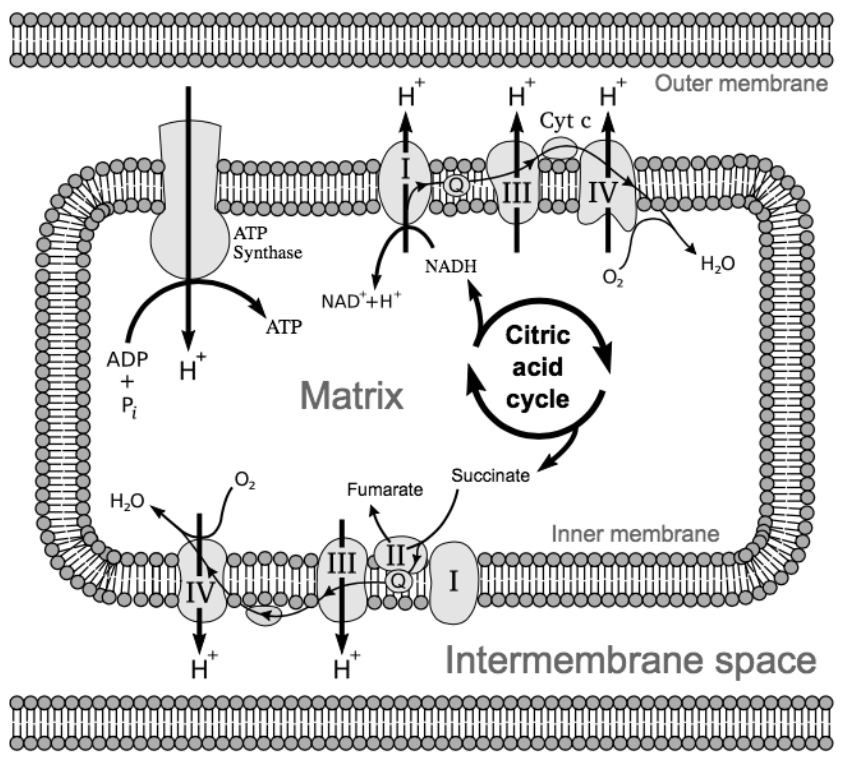
The diagram above shows the interaction between the citric acid cycle and the electron transport chain inside the mitochondrion. There are two functioning electron transport chains and a single ATP synthase that uses the \(\ce{H+}\) gradient established by those \(\ce{H+}\) pumps to make ATP.
Chemiosmosis
Why are the protein complexes pumping \(\ce{H+}\) into the intermembrane space? The intermembrane space is relatively small. As more \(\ce{H+}\) are added to this area, the intermembrane space becomes increasingly positively charged, while the matrix becomes increasingly negatively charged. This is similar to how a battery stores energy--by creating an electrochemical gradient. The positive charges repel each other and would “prefer” to be balanced across both sides of the membrane. However, they cannot directly pass through the membrane. Even though they are small, \(\ce{H+}\) ions carry a full charge, making them too polar to pass through the nonpolar tails of the phospholipid bilayer that composes the mitochondrial membranes.
An enzyme called ATP synthase allows the \(\ce{H+}\) to move back into the matrix. This enzyme is structured much like a waterwheel or turbine -- the flow of protons through the enzyme physically rotates it, converting the potential energy stored in the electrochemical gradient into kinetic energy (movement)! This kinetic energy is used to force another phosphate group onto ADP, converting the kinetic energy back into chemical energy, which is stored in the bonds of ATP
Experiment: Understanding the Relationship Between Cellular Respiration and Photosynthesis
- Put 50 mL of tap water into a flask (Flask A) and add a pH indicator, such as phenol red. Record the initial color of the solution in the table below.
- Get out a stopwatch and have a partner ready to time you. When they say go, use a straw to blow into the water + indicator. As soon as it changes color, your partner should stop the stopwatch and you should stop blowing through the straw. Record the time below.
- Repeat step 1 to set up another flask (Flask B).
- This time, before you blow into the straw, do 30 seconds of intense exercise, such as running around outside, jumping jacks, burpees, or something else fun. As soon as you are done, have your partner time you as you blow through the straw into the new flask. Stop as soon as the color changes and record the time below.
- Put a sprig of Elodea (or other aquatic plant) into Flask B, cork both flasks so no gases can get in or out, and set them both under a light source.
- Observe these flasks over the course of the lab and make note of any color changes.
|
Initial Color |
Time to Change |
Changes Under Light Source? |
|
|---|---|---|---|
|
Flask A |
|||
|
Flask B |
Why did the color of the indicator change when you blew into the liquid? What caused the change?
Did the pH of the solution go up or down?
Was there any difference in timing of color change between your two trials? If so, what might explain this difference?
Explain what you observed after you added the plant to Flask B.


