21.6: Biosynthesis of Isoprenoids
- Page ID
- 81288
By William (Bill) W. Christie and Henry Jakubowski.
This section is an abbreviated and modified version of material from the Lipid Web, an introduction to the chemistry and biochemistry of individual lipid classes, written by William Christie.
Isoprenoids: 1. Tocopherols and Tocotrienols (Vitamin E)
Tocopherols and tocotrienols constitute a series of related benzopyranols (or methyltocols) that are synthesized in plants and other photosynthetic organisms, where they have many important functions but especially as part of a complex web of antioxidants that protect plants from the activities of reactive oxygen species (ROS). First described in 1922 as a dietary factor essential to prevent fetal reabsorption in rats, it was soon understood that plants contained a fat-soluble vitamin (vitamin E) that is essential for innumerable aspects of animal development. Of these many related molecules, only one form, i.e., α‑tocopherol, is recognized as having vitamin E activity in humans in that it prevents a spectrum of human deficiency diseases termed 'ataxia', which is characterized by very low concentrations of α‑tocopherol in plasma. The progression of the disease can be prevented by the administration of α-tocopherol only, although the pathogenic mechanism appears to be uncertain. While all tocopherols are known to be powerful lipid-soluble antioxidants in vitro at least, α‑tocopherol has indirect roles in signal transduction and gene expression in animal tissues. On the other hand, specific functions (non-vitamin E) for other tocopherol forms and their metabolites in animal tissues are now being revealed. Vegetable oils are a major dietary source of vitamin E for humans.
Structure and Occurrence
In the tocopherols, the C16 side chain is saturated, and in the tocotrienols it contains three trans double bonds. Together, these two groups are termed the tocochromanols. In essence, the tocopherols have a 20-carbon phytyl tail (including the pyranol ring) and four chiral centers in total, with variable numbers of methyl groups attached to the benzene ring, while the tocotrienols have a 20-carbon geranylgeranyl tail with double bonds at the 3', 7' and 11' positions relative to the ring system. Tocopherols contain three chiral carbons, one at C2 in the chromanol ring and two in the side chain at C4′ and C8′ with R,R,R stereochemistry. The four main constituents of the two classes are termed - alpha (5,7,8-trimethyl), beta (5,8-dimethyl), gamma (7,8-dimethyl) and delta (8-methyl). In contrast to the tocopherols, the tocotrienols have only one chiral center. Plastochromanol-8 is an analog of γ-tocotrienol with a much longer side-chain. Their structures are shown in Figure \(\PageIndex{1}\).
The tocochromanols are only synthesized by plants and other oxygenic photosynthetic organisms, such as algae and some cyanobacteria, but they are essential components of the diet of animals. Of these, only natural R,R,R-α-tocopherol is now designated ‘vitamin E’, as explained below, although the other tocopherols are sometimes termed ‘vitamers’ (some claim incorrectly - nor should all forms be termed isomers strictly speaking). In the USA, the current recommended dietary allowance (RDA) is 15 mg α‑tocopherol daily for adults. In plants, there is a great range of tocochromanol contents and compositions, and photosynthetic plant tissues contain from 10 to 50 μg tocochromanols per g fresh weight. α‑Tocopherol only is present in photosynthetic membranes of plant leaves, while γ-tocopherol and other forms are found principally in fruits, seeds, and nuts. While tocopherols are present in all photosynthetic organisms, the tocotrienols are found only in certain plant families.
Seed oils are a major source for the human diet and the compositions of tocopherols in some unrefined oils are listed in Table 1. Sunflower and olive oils are good sources of α‑tocopherol and palm oil of the tocotrienols. In general, tocotrienols tend to be abundant only in seeds and fruits, especially of monocots such as wheat, rice, and barley, though a major commercial source is palm oil. In leaf tissue, α-tocopherol is often the main form, while γ-tocopherol is the primary tocopherol of many seeds. Plastochromanol-8 was first found in leaves of the rubber tree (Hevea brasiliensis) but has since been found in many other plants including rapeseed and maize, but usually at lower levels than of the tocopherols. In addition, tocopherol esters of fatty acids occur in plant tissues, where they may be an inert storage form, but unesterified tocopherols are not released during digestion in animals so they may not make a contribution to vitamin E activity.
| Table \(\PageIndex{1}\): Tocopherol and tocotrienol contents (mg/Kg) in some seed oils. | ||||||||
| α-T* | β-T | γ-T | δ-T | α-TT* | β-TT | γ-TT | δ-TT | |
|---|---|---|---|---|---|---|---|---|
| palm | 89 | - | 18 | - | 128 | - | 323 | 72 |
| soybean | 100 | 8 | 1021 | 421 | - | - | - | - |
| maize | 282 | 54 | 1034 | 54 | 49 | 8 | 161 | 6 |
| sunflower | 670 | 27 | 11 | 1 | - | - | - | - |
| rapeseed | 202 | 65 | 490 | 9 | - | - | - | - |
| * Abbreviations: T, tocopherol; TT, tocotrienol Data from: Gunstone, F.D., Harwood, J.L. and Padley, F.B. The Lipid Handbook (Second Edition) (Chapman & Hall, London)(1994). |
||||||||
An unusual tocopherol that has been termed marine-derived α-tocomonoenol is found together with α-tocopherol in a wide range of marine fish species, where it appears to be a more efficient scavenger of free radicals at low temperatures. A related isomer with a Δ11 double bond has been found in palm oil and kiwi fruit. While pumpkin seeds contain both α- and γ-tocomonoenols, other plant species contain β, γ- and δ-tocomonoenols with unsaturation in the terminal isoprene unit of the side chain. Tocochromenols or 3,4-dehydrotocopherols, i.e., with a double bond in the pyranol ring, are also known in addition to more complex tocopherol-like molecules. The structures of tocomonoenols are shown in Figure \(\PageIndex{2}\).
α-Tocopherol is a minor but ubiquitous component of the lipid constituents of animal cell membranes (non-raft domains), with estimates ranging from one molecule of tocopherol to from 100 to 1000 molecules of phospholipids, depending on the membrane. The hydrophobic tail lies within the membrane, as might be expected, and the polar head group is orientated towards the surface but below the level of the phosphate moieties of the phospholipids. There may be some limited hydrogen bonding between the hydroxyl groups and phosphate depending on the degree of hydration of the membrane. On the other hand, there is a strong affinity of α-tocopherol for polyunsaturated fatty acids, where the chromanol unit may interact with the double bonds, suggesting that tocopherol is located deep within the membrane.
α-Tocopheryl phosphate has recently been detected at low levels in plasma, liver, and adipose tissue. Its structure is shown in Figure \(\PageIndex{3}\). Together with catabolic tocopherol metabolites, it has important biological properties.
During the refining of vegetable oils, much of the natural tocopherols is lost or destroyed. Most commercial vitamin E is therefore prepared by chemical synthesis with trimethylhydroquinone and phytyl bromide as the precursors. The resulting product is a mixture of eight stereoisomers (from R,R,R- to S,S,S-methyl groups) of α-tocopherol, with the various stereoisomers differing by a factor of two in biologic activity, as a consequence of the stereochemistry of position 2 in the chromanol ring (i.e., 2S-α- compared to 2R-α-tocopherol). It is usually administered as the acetate derivative in vivo. Tocopherols are not usually regarded as effective antioxidants in the polyunsaturated seed oils of commerce, and at higher concentrations can even act as pro-oxidants, although the reasons for this are not understood.
Biosynthesis and Functions of Tocochromanols in Plants
The mechanism of biosynthesis of tocopherols has been elucidated and involves the coupling of phytyl diphosphate with homogentisic acid (2,5‑dihydroxyphenylacetic acid), followed by cyclization and methylation reactions. The plant chloroplast is the site of biosynthesis, and most of the enzymes are located on the inner membrane of the chloroplast envelope, although there is increasing evidence that plastoglobules associated with the thylakoid membrane may be involved. The pathway for biosynthesis of tocopherols is shown in Figure \(\PageIndex{4}\).
The aromatic amino acid tyrosine can be considered the basic precursor, and this is oxidized to p-hydroxypyruvic acid, which in the first committed step is converted to homogentisic acid by the enzyme p-hydroxyphenylpyruvate dioxygenase. Homogentisic acid is condensed with phytyl diphosphate, derived from phytol obtained from hydrolysis of chlorophyll, in a reaction catalyzed by a prenyl transferase to yield 2-methyl-6-phytyl-plastoquinol, which is first methylated to form 2,3-dimethyl-5-phytyl-1,4-benzoquinol and then converted by the enzyme tocopherol cyclase to γ-tocopherol. A further methylation reaction produces α-tocopherol, while modifications to the pathway produce β- and δ-tocopherols, together with plastoquinones and thence plastochromanol-8. Tocotrienols and tocomonoenols result from a similar series of reactions but with geranylgeranyl diphosphate and tetrahydro-geranylgeraniol diphosphate, respectively, as substrates in the condensation step. The isoprenoid precursors are synthesized in the plastid also by the non-mevalonate or 'MEP' pathway.
In plants, tocopherols are found mainly in the chloroplasts of green tissues, but they are also present in seeds, fruits, roots and tubers. They are especially important as antioxidant molecules, limiting the damage from photosynthesis-derived reactive oxygen species during conditions of oxidative stress, including high-intensity light stress, and the mechanisms for this antioxidant activity are discussed below. However, recent studies seem to suggest that they are just one of a number of different components that are involved in photoprotection. Certainly, any tocochromanol peroxy radicals formed must be converted back to the original compounds by the concerted action of other plant antioxidants, for example by ascorbate, glutathione, ubiquinol or lipoic acid, and antioxidant enzymes, including superoxide dismutase, catalase, and peroxidases. Tocopherols are essential for the control of non-enzymatic lipid peroxidation during seed dormancy and germination of seedlings. In their absence, elevated levels of malondialdehyde and phytoprostanes are formed, and there can be inappropriate activation of plant defense responses.
There is evidence that tocopherols play a part in intracellular signaling in plants in that they regulate the amounts of jasmonic acid in leaves, via modulating the extent of lipid peroxidation and gene expression, and so influence plant development and stress responses. Thus, by controlling the degree of lipid peroxidation in chloroplasts (redox regulation), they limit the accumulation of lipid hydroperoxides required for the synthesis of jasmonic acid, which in turn regulates the expression of genes that affect a number of abiotic stress conditions, including drought, salinity and extremes of temperature. The translocation of enzymes to the plasma membrane is regulated by tocopherols, possibly by modulating protein-membrane, altering membrane microdomains (lipid rafts), or by competing for common binding sites within lipid transport proteins. In addition, tocopherols are required for the development of the cell walls in phloem transfer cells under cold conditions. It appears that α- and γ‑tocopherol and the tocotrienols may each have distinct functions. For example, γ-tocopherol is reportedly more potent than α-tocopherol in protecting plants from the harmful effects of osmotic stresses and is important for the longevity of seeds. Efforts are underway to increase the tocopherol levels in plants by selective breeding and genetic manipulation with the aim of producing crops with greater potential health benefits to consumers and perhaps for the plants per se.
Tocopherols Metabolism in Animals
In animals, the first step in the digestion of tocopherols is their dissolution with other lipids in mixed micelles in the intestines. All tocopherol forms are absorbed to a similar extent in the intestines by means of transporters in the enterocyte apical membrane that have a broad specificity for hydrophobic molecules, such as cholesterol, vitamin D, and carotenoids. These include scavenger receptor class B type I (SR-BI), the CD36 protein, and NPC1-like intracellular cholesterol transporter 1 (NPC1L1). However, some passive diffusion cannot be ruled out. Transport across the enterocyte may involve cytoplasmic transporters or clathrin-coated vesicles before the tocopherols are incorporated into chylomicrons in free form in the Golgi apparatus for release into the lymph. At the liver, α-tocopherol specifically is taken up from the chylomicrons by a receptor-mediated mechanism with the aid of a specific tocopherol-binding protein (the α-tocopherol transfer protein (α-TTP)), i.e., a 30,500 Da cytosolic protein that has a marked affinity for α-tocopherol and can enhance its transfer between membranes. This recognizes α-tocopherol by the three methyl groups and hydroxyl on the chromanol ring and by the structure and orientation of the phytyl side chain. It is the chief regulator of whole body α-tocopherol status and is expressed primarily in the cytosol of hepatocytes in the liver, but has been reported (in much lower concentrations) in other tissues, such as the placenta.
α-TTP ultimately regulates the egress of α-tocopherol selectively from hepatocytes with the aid of the ATP-binding cassette proteins ABCA1 and ABCG for conveyance in the plasma lipoproteins, mainly in the very-low-density lipoproteins or VLDL (and thence to LDL) and HDL in humans, to the peripheral tissues (together with much smaller amounts of γ-tocopherol). Most of the other tocopherol forms are directed toward catabolism. Once in the circulation, tocopherol can exchange spontaneously between membranes and lipoproteins, and no specific transport protein for vitamin E in plasma has yet been described. Transfer of tocopherols from the VLDL to peripheral tissues occurs as triacylglycerols are hydrolyzed by the enzyme lipoprotein lipase, while that in LDL is processed via the LDL receptor-mediated uptake pathway. Within cells of peripheral tissues, including the central nervous system, α-TTP functions in transporting α-tocopherol to wherever it is required in membranes, a process that appears to be aided by phosphatidylinositol metabolites. In the brain, tocopherol is transported by apo-E rich lipoproteins. Concentrations of tocopherols can vary appreciably amongst tissues, with most in adipose tissue and adrenals, less in kidney, heart, and liver, and least in the erythrocytes.
The "α-tocopherol salvage pathway" is partly due to this process and partly to selective oxidation (see below), and the result is a 20- to 30-fold enrichment of α‑tocopherol in plasma (average concentration 22-28 μM) relative to the other tocopherols. Thus, the process of conservation of one specific tocopherol appears to determine the relative vitamin E activities of the tocopherols and tocotrienols in vivo, rather than their individual potencies as antioxidants as measured in model systems in vitro. Only α‑tocopherol (including synthetic material) or natural mixtures containing this can be sold under the label 'Vitamin E'. γ‑Tocopherol is the second most abundant form in plasma, and it is present in relatively greater proportions in the skin, adipose tissue, and skeletal muscle, where it has some specific biological properties that are distinct from those of α-tocopherol. Although tocotrienols are more potent antioxidants in vitro, they are not usually detected in tissues, although they are believed to have some important functions.
Catabolism: The unwanted surplus of tocochromanols other than α-tocopherol may be excreted in the urine and feces in the form of carboxy-chromanols, including the so-called 'Simon metabolites' - tocopheronic acids (carboxyethylhydroxychromans, CEHC) and tocopheronolactones, after oxidative cleavage of much of the phytyl tail, although these are normally detected in the form of conjugates as sulfate or glucuronidate esters, the forms in which they are excreted in feces and urine. For example for illustrative purposes in liver cells, the first step in the catabolism of γ-tocopherol is ω-hydroxylation by cytochrome P450 (CYP4F2) at the 13' carbon to form γ-13'-hydroxychromanol in the endoplasmic reticulum, followed by ω-oxidation in the peroxisomes to produce γ‑13'‑carboxychromanol, and finally by stepwise β‑oxidation in the mitochondria to cut off two or three carbon moieties from the phytyl chain in each cycle. These steps are shown in Figure \(\PageIndex{5}\).
Various carboxychromanol intermediates have been identified for all of the tocopherols together with forms in which the hydroxyl group is sulfated in human cell cultures in vitro; sulfated carboxychromanols are the main tocopherol metabolites in the plasma of rodents. As the vitamin E ω-hydroxylase has a high affinity for the tocopherols other than the α-form and does not attack that bound to the α-tocopherol transfer protein, this provides a further specific enhancement of the α‑tocopherol concentration in plasma relative to the others. Some of these catabolic metabolites may have some biological activity in their own right. For example, carboxyethylhydroxychromans derived from γ-tocopherol were reported to induce apoptosis in cancer cells and to have anti-inflammatory effects by inhibition of cyclooxygenases and 5-lipoxygenase (see below). Tocotrienols are catabolized in a similar manner, but with additional steps in which the double bonds are reduced prior to oxidation; the final carboxyethylchromanols are the same as for tocopherols.
Tocopherols as Antioxidants
Although the syndrome associated with a lack of vitamin E in the diet of animals has been known for decades, the mode of action and specific location of tocopherols in cell membranes are not clearly understood. Several theories have been proposed to explain the functions of vitamin E in animal cells. From studies in vitro, it has long been believed that a major task is to act as an antioxidant to inhibit, decrease, delay, or prevent oxidative damage to unsaturated lipids or other membrane constituents and thence to tissues by scavenging free radicals. For example, vitamin E administration can prevent lipid peroxidation and hepatotoxicity upon exposure to the free radical-generating agent carbon tetrachloride. Lipid peroxidation is also a cause of ferroptosis, an iron-dependent form of nonapoptotic cell death. However, tocopherols have functions other than as antioxidants. In non-biological systems such as foods, cosmetics, pharmaceutical preparations, etc., tocopherols are invaluable as antioxidant additives.
Because of their lipophilic character, tocopherols are located in the membranes or with storage lipids where they may be available immediately to interact with lipid hydroperoxides, such as those described in more detail in our web pages on isoprostanes, reactive aldehydes, and oxidized phospholipids. In brief, Reactive Oxygen Species (ROS), of which innumerable forms, exist can be derived by enzymatic or non-enzymatic means and produce superoxide anions and other peroxyl radicals. Superoxide radicals (O2•-) ultimately generate highly toxic hydroxyl (•OH) or alkoxyl radicals, which can abstract a hydrogen atom from bis-allylic methylene groups of polyunsaturated fatty acids under aerobic conditions in vivo in animals and plants to generate lipid peroxyl radicals (LOO•) and hydroperoxy-fatty acids. Singlet oxygen (1O2 or O=O) is an especially important ROS (non-radical) in photosynthetic tissues of plants. As radical generation is not enzymatic, all methylene groups between two cis double bonds can potentially be involved in the reaction, although not necessarily to the same degree. Tocopherols react rapidly in a non-enzymic manner unlike many other cellular antioxidants, which are dependent on enzymes, to scavenge lipid peroxyl radicals, i.e., the chain-carrying species that propagate lipid peroxidation. In model systems in vitro, all the tocopherols (α > γ > β > δ) and tocotrienols are good antioxidants, with the tocotrienols being the most potent.
In general, the oxidation of lipids is known to proceed by a chain process mediated by such free radicals, in which the lipid peroxyl radical serves as a chain carrier. In the initial step of chain propagation, a hydrogen atom is abstracted from the target lipid by the peroxyl radical as shown in Figure \(\PageIndex{6}\).
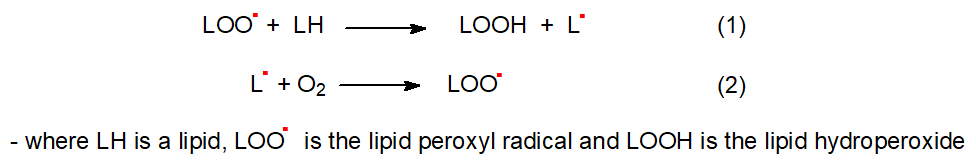
The main function of α-tocopherol is to scavenge the lipid peroxyl radical before it is able to react with the lipid substrate as shown in Figure \(\PageIndex{7}\).
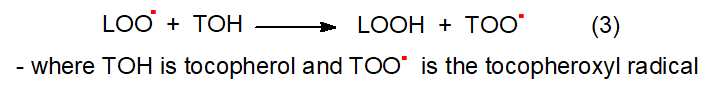
The potency of an antioxidant is determined by the relative rates of reactions (1) and (2). When a tocopheroxyl radical is formed, it is stabilized by the delocalization of the unpaired electron about the fully substituted chromanol ring system rendering it relatively unreactive, thus preventing propagation of the chain reaction. This also explains the high first-order rate constant for hydrogen transfer from α-tocopherol to peroxyl radicals, as studies of the relative rates of chain propagation to chain inhibition by α-tocopherol in model systems have demonstrated that α-tocopherol is able to scavenge peroxyl radicals much more rapidly than the peroxyl radical can react with a lipid substrate.
In biological systems, oxidant radicals can spring from a number of sources, including singlet oxygen, alkoxyl radicals, superoxide, peroxynitrite, nitrogen dioxide, and ozone. α-Tocopherol is most efficient at providing protection against peroxyl radicals in a membrane environment.
The reaction of the tocopheroxyl radical with a lipid peroxyl radical, as illustrated, yields 8α-substituted tocopherones, which are readily hydrolyzed to 8α-hydroxy tocopherones that rearrange spontaneously to form α-tocopherol quinones. In an alternative pathway, the tocopheroxyl radical reacts with the lipid peroxyl radical to form epoxy-8α-hydroperoxytocopherones, which hydrolyze and rearrange to epoxyquinones. Tocopherol dimers and trimers may also be formed as minor products. These reactions are shown in Figure \(\PageIndex{8}\).
Free radical-mediated lipid peroxidation is the major pathway of lipid oxidation taking place in humans, and α-tocopherol is a major antioxidant, but it does not scavenge the nitrogen dioxide radical, carbonate anion radical, and hypochlorite efficiently. Vitamin E forms with an unsubstituted 5-position, such as γ-tocopherol, are an exception to the rule that the various tocopherols have similar antioxidant properties in that they are able to trap electrophiles, including Reactive Nitrogen Species (RNS), which are enhanced during inflammation. The enzyme nitric oxide synthase is capable of continuously producing a large amount of nitric oxide (NO•), which can react with superoxide to produce peroxynitrite (ONOO-), a potent and versatile oxidant that can attack a wide range of biological targets. It induces lipid peroxidation and nitrates aromatic compounds and unsaturated fatty acids while isomerizing cis-double bonds in fatty acids to the trans-configuration. γ-Tocopherol is superior to α‑tocopherol in detoxifying the NO2 radical and peroxynitrite with formation of 5-nitro-γ-tocopherol, as shown in Figure \(\PageIndex{9}\).
Figure \(\PageIndex{xx}\):
This occurs in vivo, and the concentrations of 5-nitro-γ-tocopherol have been shown to be elevated in the plasma of subjects with coronary heart disease and in carotid-artery atherosclerotic plaque.
In plant and animal tissues, tocopherols can be regenerated from the tocopheroxyl radicals in a redox cycle mediated by a number of endogenous antioxidants, including vitamins A and C and coenzyme Q, and this must greatly extend their biological potency. Vitamin C (ascorbic acid) may be especially important in aqueous systems, although it may also act at the surface of membranes, to regenerate α-tocopherol, while in turn being oxidized to dehydroascorbic acid. This can be regenerated to the reduced form by glutathione (GSH) with the production of glutathione disulfide (GSSG), which can subsequently be enzymatically reduced by glutathione reductase with NAD(P)H as a cofactor. In plants, an NAD(P)H-dependent quinone oxidoreductase is involved at an early stage of the regeneration process, while tocopherol cyclase, an enzyme involved in the biosynthesis of tocopherols, re-introduces the chromanol ring. These linked cycles (the antioxidant network) are shown in Figure \(\PageIndex{10}\).
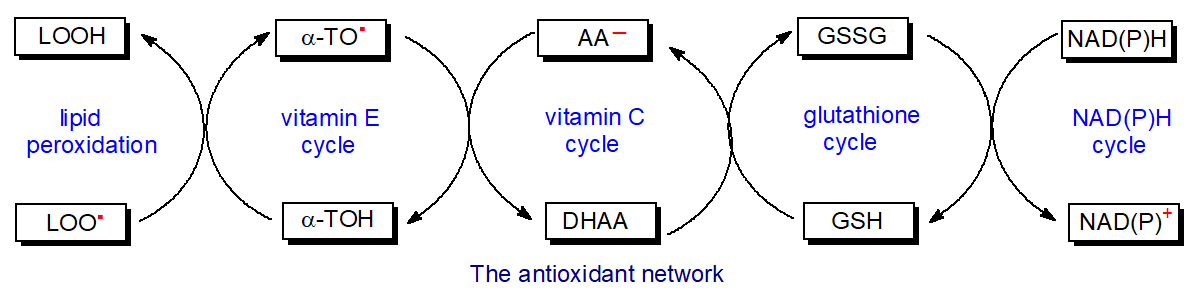
Thus, tocopherols are only one component of a complex web of metabolites and enzymes in tissues that have antioxidant activities and act by various mechanisms, including the stimulation of genes involved in signaling responses to environmental stresses. One antioxidant mechanism involves the removal of free radicals and reactive species by enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, while electron donors, such as glutathione, tocopherols, ascorbic acid, vitamin K, coenzyme Q, and thioredoxin, scavenge free radicals also. Metal-binding proteins such as transferrin, metallothionein, haptoglobin, and ceruloplasmin have antioxidant activity by sequestering pro-oxidant metal ions, such as iron and copper, although some metals such as selenium and zinc are in fact antioxidants. Other antioxidants, including flavonoids, carotenoids, and phenolic acids in addition to tocopherols, enter animal tissues via the food chain. Although the discussion here has been limited to the effects upon lipids, free radicals can cause damage to proteins, DNA, and indeed virtually any native substance in living organisms.
Biological Functions of Tocochromanols in Animals
Vitamin E deficiency has been detected in patients with fat malabsorption, cystic fibrosis, Crohn's disease, liver disease, and pancreatic insufficiency, and in premature infants. Impairment of the normal functions of the immune system has been demonstrated in animals and humans in vitamin E deficiency, and this can be corrected by vitamin E repletion. It also displays activity against nonalcoholic hepatosteatosis. Although there are various proposals for the pathogenic mechanism, none as yet appears to be generally accepted. After the discovery of the effects of vitamin E on fertility in studies with laboratory animals, its importance was documented for the development of tissues and organs such as brain and nerves, muscle and bones, skin, bone marrow, and blood, most of which are specific to α-tocopherol. However, there is no evidence for an effect of vitamin E on fertility in humans, as was originally found in the rat. The rare genetic disorder “Ataxia with Isolated Vitamin E Deficiency” or “AVED” is the result of mutations in the gene coding for α-TTP. It is caused by the death of cerebellar Purkinje cells, but administration of α-tocopherol prevents this and the subsequent development of clinical symptoms of the disease.
There appears to be little doubt that tocopherols inhibit many of the enzymes associated with inflammation in vitro in animals, and may contribute to the amelioration and treatment of some chronic diseases. However, it has been argued that data on the effects of vitamin E on biomarkers of oxidative stress in vivo are inconsistent. Oxidized metabolites of vitamin E, i.e., that have reacted as antioxidants, are barely detectable in tissues, and vitamin E maintenance in vivo does not appear to have been clearly associated with its regeneration. There appear to be significant differences between results obtained in studies with laboratory animals in comparison to those in humans. Thus, suggestions that dietary supplements of vitamin E may reduce the rate of oxidation of lipids in low-density lipoproteins in humans and thence the incidence or severity of atherosclerosis have not been confirmed by clinical intervention studies, although benefits in some conditions have been claimed. Indeed, there are suggestions that excessive vitamin E supplementation may even be harmful. One study has suggested that relatively high doses of natural α-tocopherol over a long period are required to demonstrate a significant reduction in the levels in the urine of F2 isoprostanes, which are considered to be the most reliable marker for oxidative stress in vivo. While there are many fat-soluble antioxidants in the diet, only α-tocopherol is a vitamin. It has even been suggested that tocopherol may be protected from functioning as an antioxidant in some tissues in vivo through a network of cellular antioxidant defenses, such that tocopherols are utilized only when other antioxidants are exhausted, although there is no experimental proof of this hypothesis.
At the cellular level, RRR-α-tocopherol has been shown to inhibit protein kinase C, and in the process, it inhibits the assembly and radical-producing activity of NADPH oxidase in monocytes. Similarly, vitamin E suppresses the expression of xanthine oxidase, a source of reactive oxygen species, in the liver. It is thus possible that α-tocopherol is able to diminish the levels of free radicals by preventing their production and not by scavenging them. Its physical presence in membranes adjacent to polyunsaturated fatty acids may thus limit autoxidation.
With the discovery that the antioxidant effects of various tocopherols and tocotrienols have little relation to their vitamin E activities in vivo has come the realization that they have other functions in tissues, most of which are specific to α-tocopherol. Most current research is concerned with how vitamin E and its metabolites act in signaling and controversially in the regulation of gene activity. While it is certainly true that most other vitamins are essential cofactors for specific enzymes or transcription factors, no receptor that binds specifically to vitamin E has yet been discovered. By preventing the increase of peroxidized lipids that alter both metabolic pathways and gene expression profiles within tissues and cells, it may act indirectly as a regulator of genes connected with tocopherol catabolism, lipid uptake, collagen synthesis, cellular adhesion, inflammation, the immune response and cell signaling. Vitamin E affects a number of transcription factors in this manner, including peroxisome proliferator-activated receptor gamma (PPARγ), nuclear factor erythroid-derived 2 (NRF2), nuclear factor kappa B (NFκB), RAR-related orphan receptor alpha (RORα), estrogen receptor beta (ERβ), and the pregnane X receptor (PXR).
α-Tocopherol and its metabolites are believed to modulate the activity of several enzymes involved in signal transduction, including protein kinases and phosphatases, lipid kinases and phosphatases, and other enzymes involved in lipid metabolism, but especially those with inflammatory properties such as lipoxygenases, cyclooxygenase-2, and phospholipase A2. While the credentials of tocopherols as antioxidants in vivo have been doubted, this does not preclude a role in the inhibition of oxidative enzymes, especially in relation to the function of the immune system. For example, vitamin E regulates T cell function directly by its effects upon T cell membrane integrity, signal transduction, and cell division, and it also functions indirectly by affecting eicosanoids and related inflammatory mediators generated from other immune cells. Various tocopherols and tocotrienols have been shown to suppress COX-2 involvement in prostaglandin (PGD2 and PGE2) synthesis in lipopolysaccharide-activated macrophages.
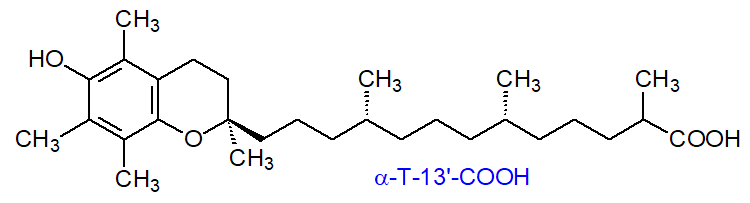
In addition, it has been established that the 13'-carboxy metabolite of α-tocopherol (α-T-13'-COOH) and other tocopherol ω-carboxylates are potent allosteric inhibitors of 5-lipoxygenase, a key enzyme in the biosynthesis of the inflammatory leukotrienes. α-T-13'-COOH accumulates in immune cells and inflamed exudates both in vitro and in vivo in mice, and it has even been suggested that the immune regulatory and anti-inflammatory functions of α-tocopherol depend on this endogenous metabolite. The structure of α-T-13'-COOH is shown in Figure \(\PageIndex{11}\).
α-Tocopherol has a stimulatory effect on the dephosphorylation enzyme, protein phosphatase 2A, which cleaves phosphate groups from protein kinase C, leading to its deactivation. The mechanism may involve the binding of vitamin E directly to enzymes in order to compete with their substrates, or it may change their activities by redox regulation. It may also compete for common binding sites within lipid transport proteins, and so may alter the traffic of lipid mediators indirectly with effects upon their signaling functions and enzymatic metabolism. For example, it binds to albumin as well as to a specific α-tocopherol-associated protein (TAP), and in the latter form especially it inhibits the phosphoinositide 3-kinase. It has been suggested that vitamin E may have a secondary role in stabilizing the structure of membranes, or it may interact with enzymes in membranes to interfere with binding to specific membrane lipids, or it may affect membrane microdomains such as lipid rafts.
Evidence suggests that the biological activities of β-, γ- and δ-tocopherols do not reflect their behavior as chemical antioxidants, but anti-inflammatory, antineoplastic, and natriuretic actions have been reported. Some non-antioxidant effects of γ-tocopherol in tissues in relation to reactive nitrogen oxide species have been observed, but the specificity of these in vivo is not yet certain. In addition, anti-inflammatory properties have been described that have been attributed to a chain-shortened metabolite. Beneficial effects against cancer cells in vitro have been observed that have been ascribed to scavenging of reactive nitrogen species, since such effects are not seen with α-tocopherol. On the other hand, vitamin E and its derivatives are believed to regulate tumor cells by activating the mitogen-activated protein kinase (MAPK) signaling pathway.
Tocotrienols have been shown to have neuroprotective effects and to inhibit cholesterol synthesis. They reduce the growth of breast cancer cells in vitro, possibly by influencing gene expression by interaction with the estrogen receptor-β. When administered in combination with either standard antitumor agents as in chemotherapy or with natural compounds with anticancer activity, they are reported to exert a synergistic antitumor effect on cancer cells. γ-Tocotrienol is reported to be an inducer of apoptosis via endoplasmic reticulum stress, while α-tocotrienol may be neuroprotective by inhibition of lipoxygenase activity. Although anti-obesity and anti-diabetic effects have been observed in mice, clinical trials with humans appear to have given inconclusive results. These properties are largely distinct from those of the tocopherols, and the pharmaceutical potential of tocotrienols against cancer, bone resorption, diabetes, and skin, cardiovascular and neurological diseases are currently being studied.
The biological functions of α-tocopheryl phosphate are slowly being revealed. In addition to being a possible storage or a transport (water-soluble) form of tocopherol, it is involved in cellular signaling and regulates a number of genes, including those involved in angiogenesis and vasculogenesis, in a different manner from α‑tocopherol per se. As it lacks the free hydroxyl group, it cannot act directly as an antioxidant, and some consider it to be the biologically active form of the vitamin. It is certainly more active in a number of biological systems in vitro than α-tocopherol, so these effects cannot be ascribed to the hydrolyzed molecule, and in some instances, it is antagonistic to α-tocopherol, for example in its activity towards phosphatidylinositol 3-kinase. On the other hand, activation requires a kinase, while a phosphatase is needed to make the system reversible, but neither has yet been identified. Synthetic phosphate derivatives of γ-tocopherol and α-tocopheryl succinate are known to have potent anti-cancer properties.
Isoprenoids: 2. Retinoids (Vitamin A)
That a dietary factor was involved in visual acuity was known to the ancient Egyptians and Greeks, but it was the 1930s before the importance of the carotenoids and their metabolites was recognized, and β-carotene and retinol were fully characterized. It is now recognized that vitamin A activity now resides in the metabolites retinol, retinal and retinoic acid, and in several provitamin A carotenoids, most notably β-carotene. A share in the Nobel Prize for Medicine in 1967 was awarded to George Wald, who over many years showed how retinol derivatives (named for their function in the retina) constituted the chemical basis of vision. Now, it is recognized that retinol, retinoic acid, and their many metabolites have innumerable other functions in human metabolism from embryogenesis to adulthood, including growth and development, reproduction, cancer, and resistance to infection. They are important natural antioxidants with benefits to health, although some potentially harmful properties have been reported.
Carotenoids are a class of highly unsaturated terpenoids that occur in innumerable molecular forms (>1000). They are common colorful pigments of plants, fungi, and bacteria, of vital importance to photosynthesis, and as dietary constituents, they can add ornament to some animal species. Apart from acting as precursors of retinoids, carotenoids per se appear to have a relatively limited range of functions in animal tissues, but they are important to vision and as antioxidants, especially in the skin. They do of course have important functions in plants and lower organisms where they originate, but this topic can only be dealt with briefly here. Other fat-soluble vitamins tocopherols (vitamin E), vitamin K, and vitamin D are discussed elsewhere.
Occurrence and Basic Metabolism of Carotenoids and Retinoids
The term ‘vitamin A’ is used to denote retinol (or all-trans-retinol, sometimes termed 'vitamin A1'), together with a family of biologically active C20 retinoids derived from this ('vitamers'). The structure of retinol is shown in Figure \(\PageIndex{12}\).
These are only found in animal tissues, where they are essential to innumerable biochemical processes. However, they cannot be synthesized de novo in animals and their biosynthetic precursors are plant carotenoids with a β-ionone ring (provitamin A), C40 tetraterpenes of which β‑carotene is most the efficient; it is an orange-red pigment that occurs in the photosynthetic tissues of plants and in seed oils. In the human diet in the developed world, plant sources tend to be less important than those from dairy products, meat, fish oils, and margarines, which provide vitamin A per se, although carrots and spinach are good sources of the provitamin. In the U.K., for example, all vegetable spreads must be supplemented with the same level of vitamin A (synthetic retinol or β-carotene) as is found in butter. While most research effort has been focused on retinoids, there is increasing interest in the biological activities of intact carotenoids in animal tissues.
The biosynthesis of carotenoids in plants via isopentenyl diphosphate and dimethylallyl diphosphate has much in common with that of the plant sterols, but this is too specialized a topic to be treated at length here. They have many important functions in plants, for example during photosynthesis or as precursors of plant hormones, and these are discussed below. Some crop plants with increased carotene levels are available with the aim of preventing vitamin A deficiency in the populations of developing countries, and further efforts are underway. Non-photosynthetic bacteria produce a different range of carotenoids, some with chain lengths other than C40 (C30 to C50).
In animals (including humans), dietary carotenoids such as β-carotene are solubilized with other dietary lipids in mixed micelles with the aid of bile acids, and they are absorbed in the intestines in intact form by a process facilitated by specific receptor proteins. Dietary retinol and retinol esters are absorbed similarly in the intestines, but the latter are first hydrolyzed by pancreatic lipase. Conversion to retinoids leading ultimately to retinol esters occurs in the enterocytes, where dietary β-carotene is subjected to oxidative cleavage at its center, the first step of which is catalyzed by a cytosolic enzyme β-carotene-15,15'-oxygenase‑1 (BCO1), which is specific for carotenes with a β-ionone ring, to yield two molecules of all-trans-retinal, which is reversibly reduced by a retinol reductase to retinol. Xanthophyll carotenoids are absorbed without cleavage mainly. These reactions are shown in Figure \(\PageIndex{13}\).
jbc: https://www.jbc.org/article/S0021-92...842-6/fulltext
Based on the incorporation of 18O into the products. it appears that the enzyme that introduces the oxygen atom, β-carotene-15,15'-oxygenase‑1 (BCO1), into the cleavage products is a dioxygenase as both atoms of oxygen in dioxygen are incorporated into products. This is in contrast to a possible mechanism in which only one oxygen atom from dioxygen is added, with the other coming from H2O if the enzyme acted as a monooxygenase. Figure \(\PageIndex{14}\) shows how oxygen could be introduced in the reaction catalyzed by BCO1 through both possible mechanisms.
The dioxygenase mechanism on the right best accounts for the incorporation of 18O isotope of oxygen in dioxygen.
Figure \(\PageIndex{15}\) shows an interactive iCn3D model of the apocarotenoid cleavage oxygenase from Synechocystis, a Retinal-Forming Carotenoid Oxygenase (2BIW)
Figure \(\PageIndex{15}\): Retinal-Forming Carotenoid Oxygenase from Synechocystis (2BIW). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov...dttF958wFPAUP7
The enzyme has a bound carotene analog, (3R)-3-hydroxy-8'-apocarotenol. It contains an active site Fe2+ ion at the end of a hydrophobic tunnel. The ion is ligated by 4 histidine side chains. On binding, three of the trans C=C bonds convert to a distorted cis-trans-cis conformation. The middle trans bond is proximal to the Fe2+ which is ligated by dioxygen, the source of the incorporated oxygen atoms on cleavage.
Any unchanged β-carotene and newly formed retinol esters in the enterocytes are incorporated into chylomicrons and released into the lymphatic system and thence into the bloodstream, where some is taken up by peripheral tissues before most is absorbed by the liver. Some intact carotene and other carotenoids are transferred to lipoproteins (LDL and HDL) for transport in plasma, with assistance from specific binding and transport proteins, and for example, carotene can be absorbed at the placental barrier and transferred to the fetus for conversion to retinoids that are essential for development. Within the hepatocytes, retinol esters are hydrolyzed in the late endosomes with the release of free retinol into the cytosol, from which it can be released back into the circulation, converted to retinoids or transferred to hepatic stellate cells for storage in lipid droplets, the main body reservoir of vitamin A. In these specialized cells, retinol is esterified to form retinyl palmitate by the transfer of fatty acids from position sn-1 of phosphatidylcholine, mainly via the action of a membrane-bound lecithin:retinol acyltransferase (LRAT) in the endoplasmic reticulum. There are also lesser acyl-CoA dependent pathways, including an acyl CoA:retinol acyltransferase and even the enzyme diacylglycerol acyltransferase 1 (DGAT1); esterification is facilitated by binding to cellular retinol-binding protein type II (CRBP2).
Both retinol and retinoic acid are precursors of a number of metabolites (retinoids), which are required for specific purposes in tissues, by enzymatic modification of the functional groups and geometrical isomerization of the polyene chains. In the liver, activation of the retinol pathway involves first mobilization of the ester, followed by hydrolysis by retinol ester hydrolases, which includes carboxylesterase ES-10. Then, the reversible oxidation of retinol to retinal is carried out by one of several enzymes that include dehydrogenases and various cytochrome P450s, before some retinal is oxidized irreversibly to retinoic acid by enzymes with retinal dehydrogenase activity. On-demand conversion of retinol to retinoic acid occurs by the same mechanisms in other tissues, although for vision, retinol esters serve directly as the substrate for the formation of the visual chromophore 11‑cis-retinal (see below). Retinyl-β-D-glucoside, retinyl-β-D-glucuronide, and retinoyl-β-D-glucuronide are naturally occurring and biologically active metabolites of vitamin A, which are found in fish and mammals. Indeed, the last has similar activity to all-trans-retinoic acid without any of the unwanted side effects in some circumstances.
Cleavage of β-carotene at double bonds other than that in the center or of a wider range of other carotenoids occurs by the action of a related enzyme β‑carotene-9',10'-dioxygenase (β‑carotene-oxygenase‑2 or BCO2) in mitochondria, which leads to the formation of similar molecules, i.e. β-apocarotenals and β‑apocarotenones of variable chain-length. While these may exert distinctive biological activities in their own right, there is evidence that they can also be metabolized to form retinal.
In the aqueous environment within cells, as well as in plasma, retinol, retinal and retinoic acid are bound to retinoid-binding proteins (RBP), which solubilize, protect and in effect detoxify them. These proteins also have a role in facilitating retinoid transport and metabolism; some are present only in certain tissues, and many are specific for particular retinoids and metabolic pathways. To prevent infiltration through the kidneys, retinol, and holo-RBP form an association in blood with a protein termed transthyretin (TTR), which also serves as a thyroid hormone carrier and is essential for secretion. Normally, vitamin A circulates in plasma as a retinol:RBP:TTR complex with a 1:1:1 molar ratio. Unesterified retinol is the main form of the vitamin that is exported from the liver upon demand, and it is transported in the blood in this bound form in VLDL, LDL, and HDL, with some directly from the diet in the chylomicrons and their remnants. Peripheral tissues have specific receptors to take up what they require, probably after hydrolysis of any esters to retinol by means of the enzyme lipoprotein lipase. Then, retinol dissociates from the protein as it forms a complex with a receptor (STRA6) at a target cell and diffuses through the plasma membrane, a process driven by retinol esterification.
The RBP-TTR complex does not bind to retinal and retinoic acid, although these do bind to RBP on its own, and most of the low levels of retinoic acid transported in the blood are bound to albumin. Local levels of retinoic acid are the result of an interplay between enzymes of synthesis, binding, and catabolism. For example, within cells retinoic acid binding proteins (CRABP1 and CRABP2) bind to the newly synthesized retinoic acid, increase its rate of metabolism and protect cells from an excess.
In skin, 3,4-dehydroretinoids are synthesized from all-trans retinoids by the desaturase Cytochrome P450 27C1 with the assistance of cellular retinol-binding proteins (CRBPs). 3,4-Dehydroretinol, which is sometimes termed vitamin A2, is shown in Figure \(\PageIndex{16}\).
Its derivative 3,4‑dehydroretinal is used as a visual chromophore in many cold-blooded vertebrates including lampreys, fish, amphibians, and some reptiles (see below). Geranylgeranoic acid has structural similarities to retinoic acid and has been termed an acyclic retinoid, although it has no vitamin A activity. It is synthesized in animal tissues from mevalonate, and together with its 2,3‑dihydro metabolite, has potent anticancer properties.
Retinol esters: A relatively small proportion of the cellular retinoids is located in membranes in tissues. Rather, retinol esters, mainly retinyl palmitate, are the main storage form of vitamin A, and they occur in many different organs, including adipose tissue and testes, but chiefly in stellate cells of the liver and pancreas. How the retinol is directed specifically to these cells and enters them prior to esterification is not known. Although hepatic stellate cells are much smaller and less abundant than hepatocytes (only 5 to 8% of all liver cells), they are characterized by cytoplasmic lipid droplets that contain 90-95% of the hepatic retinoids (and up to 80% of the body pool) in addition to other non-retinoid lipids; the lecithin:retinol acyltransferase is the only retinol ester synthase in this instance. In addition, specialized cells in the eye store retinoids essential for vision in the form of lipid droplets. When the supply of retinol in the diet is limited, hepatic stores of retinol esters are mobilized as retinol ester hydrolases are activated to maintain constant circulating retinol levels; hormone-sensitive lipase is the most important of these enzymes, although the adipose tissue triacylglycerol lipase and the lysosomal acid lipase are also involved.
Catabolism: All-trans-retinoic acid formation is irreversible, so its synthesis and degradation must be tightly regulated. As a first step in catabolism, the excess is cleared by conversion to more polar metabolites through oxidation by various enzymes of the cytochrome P450 family. Secondly, the water-soluble retinoic acid metabolites, including 4-hydroxy-, 4-oxo- and 18-hydroxy-retinoic acids, conjugate with glucuronic acid and then can be rapidly removed from circulation and eliminated from the body via the kidney.
Retinoids and Vision
The structure of 11-cis-retinal, which we discussed in Chapter 11, is shown in Figure \(\PageIndex{17}\)
Retinoids are essential for vision, and there is now a good appreciation of how this works at the molecular level. In the eye, uptake of retinol from the circulation is mediated by the transmembrane cell-surface STRA6 receptor of the retinal pigment epithelium, a pigmented monolayer of cells located between the photoreceptors and choroid that nourishes retinal visual cells and catalyzes the release of retinol from retinol-binding proteins and transports it to the cytosol. The process by which light is converted to a signal recognized by the brain, sometimes termed the 'retinoid (visual) cycle', requires a two-cell system beginning in the retinal pigment epithelium and continuing in photoreceptor cells, i.e. retinal rod and cone cells in the eye that contain membranous vesicles that serve as light receptors. Roughly half of the proteins in these vesicles consist of the protein conjugate, rhodopsin, which consists of a protein – opsin – with the retinoid 11-cis-retinal. Each step in the visual process requires specific binding or transport proteins, and especially the interphotoreceptor retinoid-binding protein (IRBP).
All-trans-retinol is first converted to its ester by the enzyme lecithin:retinol acyltransferase as described above in the RPE, and the products coalesce into lipid droplets, i.e. dynamic organelles termed 'retinosomes'. The next step involves a dual-purpose enzyme (RPE65) in the endoplasmic reticulum, which cleaves the O-alkyl bond (not a conventional hydrolysis reaction) in the retinol ester and at the same time causes a change in the geometry of the double bond in position 11 of retinol from trans to cis. The 11-cis-retinol is then oxidized to 11-cis-retinal by 11-cis-retinal dehydrogenase (RDH5). The full cycle is shown in Figure \(\PageIndex{18}\ below.
The final part of the cycle occurs in the photoreceptor, where first the 11-cis-retinal is reacted with opsin to produce the protein conjugate rhodopsin in a protonated form. When rhodopsin is activated by light, the cis-double bond in the retinoid component is isomerized non-enzymatically by the energy of a photon to the 11‑trans form with a change of conformation that in turn affects the permeability of the membrane and influences calcium transport. This results in further molecular changes that culminate in the release of opsin and all-trans-retinal, which is the trigger that sets off the nerve impulse so that the light is perceived by the brain.
A second mechanism for 11-cis-retinal formation that may function to ensure continuous visual responsiveness in bright light involves the (RPE)-retinal G protein-coupled receptor (RGR), which can function as a retinaldehyde photoisomerase. As the enzyme RPE65 functions optimally under low light conditions, it is believed that RGR prevents the saturation of photoreceptors under high light levels, and in this way facilitates vision in daylight. The isomerase, RPE65, and the photoisomerase, RGR, operate together to provide a sustained supply of the visual chromophore under different levels of illumination.
The all-trans-retinal is removed from the photoreceptor either by reduction to all-trans-retinol by all-trans-retinol dehydrogenase 8 expressed in the outer segments of photoreceptors or after transport by means of a specific transporter (ABCA4), which provides phosphatidylethanolamine (PE) for conversion to the Schiff-base adduct, i.e. N-retinylidene-phosphatidylethanolamine, as shown in Figure \(\PageIndex{19}\).
It flips from the lumen to the cytosolic leaflet of the disc membrane. This process prevents non-specific aldehyde activity with the effect of removing potentially toxic retinoid compounds from the photoreceptors. The adduct is a transient sink that dissociates so the retinal can be reduced back to all-trans-retinol by the cytoplasmic retinol dehydrogenase. All-trans-retinol exits the photoreceptor and enters the retinal pigment epithelium with the aid of binding to the retinoid-binding protein (IRBP) where it is converted back to a retinyl ester to complete the cycle and restore light sensitivity.
As a side-reaction, some troublesome bis-retinoid adducts of PE (and further byproducts) may be produced by non-enzymatic mechanisms, and these can accumulate with age to affect vision. Lower organisms: Bacteriorhodopsin is the best studied of a family of opsins, found in archaea, eubacteria, fungi, and algae. It is a protein with seven transmembrane helices that acts as an opto-electrical transducer or light-gated active ion pump to capture photon energy via its covalently bound chromophore, all-trans-retinal, converting it to 13-cis-retinal, and moves protons against their electrochemical gradient from the cytoplasm to the extracellular space. In Archaea, it is known as the "purple membrane" and can occupy a high proportion of the surface area of the organism.
Other Functions of Retinoids in Health and Disease
In addition to their function in vision, it is now realized that retinoids have essential roles in growth and development, reproduction and resistance to infection. They are particularly important for the function of epithelial cells in the digestive tract, lungs, nervous system, immune system, skin, and bone at all stages of life. They are required for the regeneration of damaged tissues, including the heart, and they appear to have some potential as chemo-preventive agents for cancer and for the treatment of skin diseases such as acne. Under pathological conditions, stellate cells lose their retinoid content and transform into fibroblast-like cells, contributing to the fibrogenic response. Cirrhosis of the liver is accompanied by a massive loss of retinoids, but it is not clear whether this is a cause or a symptom, and there appears to be confusion as to when supplementation may be helpful in this and other diseases of the liver. Like retinol and retinoic acid, the metabolite 9-cis-retinoic acid also has valuable pharmaceutical properties.
With such a large number of double bonds in conjugation, it is not surprising that carotenoids in general, and retinoids in particular are efficient quenchers of singlet oxygen and scavengers of other reactive oxygen species. However, any direct antioxidant properties are not believed to be important in terms of general health in vivo, and it is not clear how relevant the physical properties of retinoids are to specific biochemical processes in comparison to their effects on signaling and gene transcription. There is a caveat that retinoids may stimulate some antioxidant genes and so have an indirect antioxidant function. In fact, nutritional studies with dietary supplements of carotenoids have sometimes suggested pro-oxidant activity. One explanation for detrimental effects may be that regeneration of the parent carotenoid or retinoid from the corresponding radical cation may be limited when concentrations of reductants such as ascorbic acid are low.
Many of the retinol metabolites function as ligands to activate specific transcription factors for particular receptors in the nucleus of the cell, and thus they control the expression of a large number of genes (>500), including those essential to the maintenance of normal cell proliferation and differentiation, embryogenesis, for a healthy immune system, and for male and female reproduction. In the innate immune system, vitamin A is required for the differentiation of cells such as macrophages, neutrophils and natural killer cells, while all-trans-retinoic acid is involved in differentiating the precursors of dendritic cells. Retinoic acid and its 9-cis-isomer are especially important in this context, and they are often considered the most important retinoids in terms of function other than in the eye. This structure of the cis-isomer is shown in Figure \(\PageIndex{20}\).
In essence, retinoic acid moves to the nucleus with the aid of small intracellular lipid-binding proteins (CRABP2 and FABP5), which channel it to specific nuclear receptors, the retinoic acid receptors (RAR) of which there are three, RAR-α, β and γ. These are ligand-dependent regulators of transcription and they function in vivo as heterodimers with retinoid X receptors (RXR) to process the retinoic acid signal by acting through polymorphic retinoic acid response elements (RAREs) within the promoter regions of responsive genes. Similarly, 9-cis-retinoic acid and 9-cis-13,14-dihydroretinoic acid are high-affinity ligands for RXR in mice. Together with retinoic acid, these are also ligands for the farnesoid X receptor (FXR), which forms a heterodimer with RXR. The latter receptor complex is involved primarily in bile acid homeostasis, and conversely, there are suggestions that bile acids may have regulatory effects on vitamin A homeostasis.
In addition, other nuclear receptors, such as the peroxisome proliferator-activated receptor PPARγ forms a heterodimer with the retinoid X receptor and is activated by retinoic acid to recruit cofactors. This complex in turn binds to the peroxisome proliferator response element (PPRE) gene promoter, leading to regulation mainly of those genes involved in lipid and glucose metabolism, including some involved in inflammation and cancer. To add to the complexity, retinoic acid has extra-nuclear, non-transcriptional effects, such as the activation of protein kinases and other signaling pathways.
It has also become evident that many of the functions of retinoids are mediated via the action of specific binding proteins (as discussed briefly above), which control their metabolism in vivo by reducing the effective or free retinoid concentrations, by protecting them from unwanted chemical attack, and by presenting them to enzyme systems in an appropriate conformation. With some tissues, retinol-bound RBP in the blood is recognized by the membrane protein STRA6, which transports retinol into cells where it binds to an intracellular retinol acceptor, cellular retinol-binding protein 1 (CRBP1), and is then able to activate a signaling cascade that targets specific genes. In addition, a specific retinol-binding protein secreted by adipose tissue (RPB4) is involved in the development of insulin resistance and type 2 diabetes, possibly by affecting glucose utilization by muscle tissue, with obvious application to controlling obesity. In the eye, the activity of retinoic acid during development is controlled by binding to apolipoprotein A1.
All-trans-retinoic acid has been shown to be effective against many different types of human cancers, especially in model systems but also in some clinical trials, because of its specific effects on cell proliferation, differentiation, and apoptosis (where its relatively low toxicity at normal tissue levels is a virtue). For example, it induces complete remission in most cases of acute promyelocytic leukemia when administered in combination with other chemotherapy techniques. Similarly, 13-cis-retinoic acid has been used successfully in the treatment of children with high-risk neuroblastoma to reduce the risk of recurrence and increase long-term survival rates. However, the efficacy of similar treatments against other types of acute myeloid leukemia and solid tumors appears to be poor. It is hoped that current efforts to obtain a better understanding of the mechanism of the anti-cancer activities will lead to improved treatments. Synthetic analogs of retinoic acid, termed rexinoids, which activate retinoic X receptors, also hold promise as anti-cancer agents.
Vitamin A deficiency in children and adult patients is usually accompanied by impairment of the immune system, leading to a greater susceptibility to infection and an increased mortality rate, often with growth retardation and congenital malformations. However, vitamin A deficiency in malnourished children is the major reason for childhood mortality in the underdeveloped world, causing over 650,000 early childhood deaths annually and pediatric blindness. This is doubly tragic in that it is so easily prevented. In adults, vitamin A deprivation affects the reproductive system, inhibiting spermatogenesis in males and ovulation in females. Unfortunately, it is not always easy to distinguish between the effects of vitamin A deficiency and primary defects of retinoid signaling.
Functions of Xanthophylls and Other Carotenoids in Humans
Xanthophylls are plant C40 tetraterpenes that differ from the carotenoids in having oxygen atoms in the ring structures (hydroxyl, oxo, or epoxyl). Lutein, zeaxanthin, and meso-zeaxanthin from dietary sources, such as green leafy vegetables and yellow and orange fruits and vegetables, are found specifically in the macula of the eye in humans and other primates, i.e. the functional center of the retina in a small central pit known as the macula lutea, where they enhance visual acuity and protect the eye from high-intensity, short-wavelength visible light. They are powerful antioxidants in a region vulnerable to light-induced oxidative stress. Binding proteins specific for lutein- and zeaxanthin mediate the highly selective uptake of these carotenoids into the retina, but meso-zeaxanthin is mainly a metabolite of dietary lutein. Macular xanthophylls decrease the risk of age-related macular degeneration. In the brain, they may stimulate and maintain cognitive function in the elderly, and assist with brain development in infants. Hydroxylated xanthophylls such as lutein, shown in Figure \(\PageIndex{21}\), occur both in the free form and esterified to fatty acids; the latter are hydrolyzed in the intestines when consumed by animals.
Many other carotenoids are absorbed from the diet, and are subject to oxidative cleavage or other catabolic processes, partly in the intestines and partly in other tissues after transport in the lipoproteins. Some carotenoids remain intact and are believed to act as antioxidants, and some may have specific anti-inflammatory actions. For example, carotenoids accumulate in the skin of mammals, where they may have an antioxidant and photo-protective role as well as effects on the moisture content, texture, and elasticity. Lycopene may have protective effects against atherogenesis, coronary heart disease, and prostate cancer.
Functions of Carotenoids in Plants
As carotenoids have a polyene chain of 9 to 11 double bonds in conjugation, they are able to absorb light in the gap of chlorophyll absorption, and so function as additional light-harvesting pigments in plants. Their distinctive arrangement of electronic levels gives them the capacity to transfer excitation energy from the carotenoid excited state to chlorophyll in the light-harvesting complex (photosystem II). Energy can also be transferred back from chlorophyll to carotenoids as a photoprotection mechanism. During photosynthesis, damaging species are produced by both light and oxygen with reactive oxygen species (ROS) of special concern. The energy is transferred from chlorophyll to the polyene tail of the carotenoid where electrons are moved between the carotenoid bonds until the most balanced or lowest energy state (state) is reached. While there is therefore appreciable potential for carotenoids to act as antioxidants in plants, it is uncertain how important this is from a practical functional standpoint. The length of the polyene tail of carotenoids determines which wavelengths of light will be absorbed by the plant, and those not absorbed are reflected and so determine coloration. F
Carotenoids are precursors for two plant hormones and a diverse set of apocarotenoids. For example, abscisic acid, shown in Figure \(\PageIndex{21}\), is a C15 isoprenoid plant hormone, which is synthesized in plastids from the C40 carotenoid zeaxanthin.
A series of enzyme-catalyzed epoxidations and isomerizations is involved followed by cleavage of the intermediate product by a dioxygenation reaction and further oxidations to yield eventually abscisic acid. Functioning via signaling cascades, abscisic acid regulates innumerable biological effects in plants, especially in relation to developmental processes that include plant growth, seed and bud dormancy, embryo maturation and germination, cell division and elongation, floral growth and the control of stomatal closure. It is critical for the responses to environmental stresses that include drought, cold and heat stress, salinity, and tolerance of heavy metal ions. Similarly, strigolactones are C15 oxidation products of carotenoids that are involved in the regulation of symbiosis between plants and arbuscular mycorrhizal fungi and in interactions with plant parasites.
Isoprenoids: 3. Other Membrane-Associated Isoprenoids
Terpenes (isoprenoids) are one of the most varied and abundant natural products produced by animals, plants, and bacteria. They are generally defined on the basis of their biosynthetic derivation from isoprene units (C5H8), with 55,000 different types characterized to date according to a recent estimate. By most definitions, all isoprenoids should be classified as ‘lipids’, from simple monoterpenes such as geraniol, which is derived from two prenol units, to complex polymers such as natural rubber. Only those isoprenoids that have a functional role in cellular membranes will be discussed here. These include plastoquinone, ubiquinone (coenzyme Q), phylloquinone and menaquinone (vitamin K), dolichol and polyprenols, undecaprenyl phosphate and lipid II, and farnesyl pyrophosphate, together with some key biosynthetic precursors. The nature and function of tocopherols and tocotrienols (vitamin E) and retinoids (vitamin A) are relevant here, but have been discussed previously. Of course, sterols are also isoprenoids.
There are two basic mechanisms for the biosynthesis of the isoprene units that are the precursors for the biosynthesis of isoprenoids, i.e., isopentenyl pyrophosphate and dimethylallyl pyrophosphate. These are the mevalonate pathway, which is located in the cytosol of the cell, and the non-mevalonate pathway, found mainly in the plastids of plants. These have been discussed previously.
Phytol
Phytol or (2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-ol, i.e., with 20 carbons in a 16-carbon chain and one double bond, is an acyclic diterpene alcohol, which is synthesized in large amounts in plants as an essential component of chlorophyll, the most important photosynthetic pigment in plants and algae. Geranylgeranyl-diphosphate synthesized in chloroplasts via the 4-methylerythritol-5-phosphate (non-mevalonate) pathway is the primary precursor of phytol following reduction of three double bonds by geranylgeranyl reductase, and this can occur before or after attachment to chlorophyll, depending upon species. Chlorophyll dephytylase (CLD1) is the enzyme in plants responsible for chlorophyll hydrolysis and the release of phytol. The reactions are shown in Figure \(\PageIndex{22}\).
Little free phytol is present in plant tissues, although some phytol esters of fatty acids may occur, especially when plants are stressed during nitrogen deprivation or in senescence, when chlorophyll is degraded, fatty acids are released from glycerolipids and a phytol ester synthase is induced as part of a detoxification and recycling process. Bell peppers and rocket salad are especially rich sources under normal conditions. In addition, phytol as its diphosphate is utilized for the synthesis of tocopherols (vitamin E) and phylloquinol (vitamin K - see below), and the precursor geranylgeraniol and its fatty acid ester occur in small amounts in some plant species. It is the biosynthetic precursor of tocotrienols and the highly unsaturated carotenoids (and hence of retinoids). Phytenal has been isolated as an intermediate in the catabolism of phytol in plants, but further steps are uncertain although phytanoyl-CoA has been detected in stressed plants. As phytenal is highly reactive and potentially toxic via its interaction with proteins, its accumulation must be kept at a low level by competing pathways.
In ruminant animals, chlorophyll is hydrolyzed by rumen microorganisms with the release of free phytol. This does not occur in humans, but some phytol may be ingested with plant foods either in free form or as phytol esters and can be absorbed from the intestines. Within animal tissues, phytol is oxidized to phytanic acid. Phytol and/or its metabolites have been reported to activate the transcription factors PPARα and retinoid X receptor. In mice, oral phytol induces a substantial proliferation of peroxisomes in many organs.
Plastoquinone
A molecule that is related to the tocopherols, plastoquinone, is found in cyanobacteria and plant chloroplasts, and it is produced in plants by analogous biosynthetic pathways to those of tocopherols in the inner chloroplast envelope with solanesol diphosphate as the biosynthetic precursor of the side chain; there appears to be a somewhat different mechanism in cyanobacteria. The molecule is sometimes designated - 'plastoquinone-n' (or PQ-n), where 'n' is the number of isoprene units, which can vary from 6 to 9. It's structure is shown in Figure \(\PageIndex{23}\).
Plastoquinone has a key role in photosynthesis, by providing an electronic connection between photosystems I and II, generating an electrochemical proton gradient across the thylakoid membrane. This provides energy for the synthesis of adenosine triphosphate (ATP). The reduced dihydroplastoquinone (plastoquinol) that results in the transfers further electrons to the photosynthesis enzymes before being re-oxidized by a specific cytochrome complex; the redox state of the plastoquinone pool regulates the expression of many of the genes encoding photosystem proteins. X-Ray crystallography studies of photosystem II from cyanobacteria show two molecules of plastoquinone forming two membrane-spanning branches. In addition, plastoquinone has antioxidant activity comparable to that of the tocopherols, protecting especially against excess light energy and photooxidative damage. Similarly, in thylakoid membranes, plastoquinol is able to scavenge superoxide with the production of H2O2. Plastoquinone is a cofactor participating in desaturation of phytoene in carotenoid biosynthesis, and the biosynthetic precursor of plastochromanols. With these many different functions, plastoquinone connects photosynthesis in plants with metabolism, light acclimation, and stress tolerance.
Plastoquinone-9, together with phylloquinone, tocopherol, and plastochromanol-8, is stored in plastoglobuli, lipoprotein-like micro-compartments, which enable exchange with the thylakoid membrane and are also involved in chlorophyll catabolism and recycling. It has been suggested that the redox state of the plastoquinone pool is the main redox sensor in chloroplasts that initiates many physiological responses to changes in the environment and in particular to those related to light intensity by regulating the expression of chloroplast genes.
Ubiquinone (Coenzyme Q)
The ubiquinones, which are also known as coenzyme Q (CoQ) or mitoquinones, have obvious biosynthetic and functional relationships to plastoquinone and they are found in all the domains of life (hence the name). They have a 2,3‑dimethoxy-5-methylbenzoquinone nucleus and a side chain of six to ten isoprenoid units; the human form illustrated below has ten such units (coenzyme Q10), i.e., it is 2,3-dimethoxy-5-methyl-6-decaprenyl-1,4-benzoquinone, while that of the rat has nine, Escherichia coli has eight and Saccharomyces cerevisiae has six. In plants, ubiquinones tend to have nine or ten isoprenoid units. Forms with a second chromanol ring, resembling the structures of tocopherols, are also produced (ubichromanols), but not in animal tissues. They are generated on an industrial scale for pharmaceutical purposes by yeast fermentation. Because of their hydrophobic properties, ubiquinones are located entirely in membrane bilayers in most eukaryote organelles, probably at the mid-plane.
Ubiquinones are synthesized de novo in mitochondria in most cells in animal, plant, and bacterial tissues by a complex sequence of reactions from the essential amino acid phenylalanine and then tyrosine to generate p-hydroxybenzoic acid, which is the key precursor that is condensed with the polyprenyl unit (from the cholesterol synthesis pathway) via a specific transferase; this is followed by decarboxylation, hydroxylation, and methylation steps, depending on the specific organism, although some of the required enzymes have yet to be fully characterized. In Escherichia coli, biosynthesis does not occur in a membrane environment as had been thought. Rather, the seven proteins that catalyze the last six reactions of the biosynthetic pathway, following the attachment of the isoprenoid tail, form a stable complex or metabolon in the cytoplasm so enabling modification of the hydrophobic substrates in a hydrophilic environment.
In mitochondria, coenzyme Q is present both as the oxidized (ubiquinone) and reduced (ubiquinol) forms as shown in Figure \(\PageIndex{24}\).
Figure \(\PageIndex{24}\): Ubiquinone-ubiquinol interconversion
Ubiquinones are essential components of the respiratory electron transport system, possibly as part of supramolecular complexes, taking part in the oxidation of succinate or NADH via the cytochrome system to generate the protonmotive force used by the mitochondrial ATPase to synthesize ATP. In this process, coenzyme Q transfers electrons from the various primary donors, including complex I, complex II, and the oxidation of fatty acids and branched-chain amino acids, to the oxidase system (complex III), while simultaneously transferring protons to the outside of the mitochondrial membrane with the result of a proton gradient across the membrane. As a consequence, it is reduced to ubiquinol. Thus, it is an essential component of the cycle that generates the proton motive force driving ATP production via oxidative phosphorylation. In yeast, one coenzyme Q binding protein (COQ10), and in humans two related proteins (COQ10A and COQ10B) may serve as chaperones or transporters during this process. Mitochondrial coenzyme Q is also implicated in the production of reactive oxygen species by a mechanism involving the formation of superoxide from ubisemiquinone radicals, and in this way is responsible for causing some of the oxidative damage behind many degenerative diseases. In this action, it is a pro-oxidant. It is most abundant in organs with a high metabolic rate such as the heart, kidneys, and liver.
In complete contrast in its reduced form (ubiquinol) in non-mitochondrial cellular membranes and plasma lipoproteins, it acts as an endogenous antioxidant, the only lipid-soluble antioxidant to be synthesized endogenously. It inhibits lipid peroxidation in biological membranes and serum low-density lipoproteins, and it may also protect membrane proteins and DNA against oxidative damage. The ferroptosis suppressor protein 1 (FSP1) replenishes ubiquinol, and this acts protectively by combating the lipid peroxidation that drives ferroptosis. The mechanism involves the recruitment of FSP1 to the plasma membrane following myristoylation, where this functions as an oxidoreductase that reduces ubiquinone to ubiquinol, which acts as a lipophilic radical-trapping antioxidant that halts the propagation of lipid peroxides. In this manner, it regulates cellular redox status and cytosolic oxidative stress, and thereby is a controlling factor in apoptosis. Other NAD(P)H dehydrogenases with CoQ reductase activity include cytochrome b5 reductase and NQo1 (NAD(P)H:quinone oxidoreductase).
Although ubiquinone has only about one-tenth of the antioxidant activity of vitamin E (α-tocopherol), it is able to stimulate the effects of the latter by regenerating it from its oxidized form back to its active fully reduced state (similarly with vitamin C). However, ubiquinones and tocopherols appear to exhibit both cooperative and competitive effects under different conditions. Similarly, in bacteria and other prokaryotes, ubiquinones participate a large number of redox reactions, notably in the respiratory electron transport system but also in other enzyme reactions that require electron donation, including the formation of disulfide bonds.
Coenzyme Q has many other functions that are not related directly to its antioxidant function. Some coenzyme Q is used in mitochondria by enzymes that link the mitochondrial respiratory chain to other metabolic pathways, including fatty acid β-oxidation as an electron acceptor, nucleotide biosynthesis de novo, amino acid oxidation (glycine, proline, glyoxylate, and arginine), and detoxification of sulfide. Via these activities, coenzyme Q may modulate metabolic pathways located outside the mitochondria indirectly. There are also suggestions that coenzyme Q may be involved in redox control of cell signaling and gene expression, and in particular to repress the expression of inflammatory genes. In relation to its anti-inflammatory properties, clinical studies suggest that supplementation with coenzyme Q10 reduces the levels of the inflammatory mediators C-reactive protein, interleukin-6, and tumor necrosis factor alpha (TNFα) to a significant degree. In addition, it is a regulator of mitochondrial permeability, and in relation to pyrimidine nucleotide biosynthesis, it is required for DNA replication and repair.
Dietary ubiquinone, i.e., that in food or dietary supplements, is absorbed by enterocytes via a process of “passive facilitated diffusion”, probably requiring a carrier molecule, before incorporation into chylomicrons for transport to the liver. This eventually leads to elevated levels of ubiquinol in blood, especially in the LDL and VLDL lipoproteins, presumably because of the reduction of the oxidized form in the lymphatic system. In consequence, there is reported to be enhanced protection against lipid peroxidation with beneficial effects on health, especially in relation to cardiac function, sperm motility, and neurodegenerative diseases. For example, CoQ levels in both plasma and the heart correlate with heart failure in patients, and clinical trials of dietary supplementation have shown promising results. This may be of particular importance in the elderly or in patients on statins, when endogenous synthesis declines. A CoQ10 deficiency syndrome is associated with inherited pathological diseases, defined by a decrease of the CoQ10 content in muscle and/or cultured skin fibroblasts. Early clinical trials with CoQ10 and a synthetic analog, idebenone, against various neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and others, are encouraging.
Phylloquinone and Menaquinones (Vitamin K)
Phylloquinone or 2-methyl-3-phytyl-1,4-naphthoquinone is synthesized in the inner chloroplast envelope of cyanobacteria, algae, and higher plants by a mechanism analogous to that of the tocopherols, i.e., from chorismate in the shikimate pathway with a prenyl side chain derived from phytyldiphosphate. In this membrane, it is a key component of the photosystem I complex where it receives an electron from the chlorophyll a acceptor molecule and then donates an electron to the membrane-associated iron-sulfur protein acceptor cluster in the complex. In an obvious parallel to the plastoquinones (above), two molecules of phylloquinone form two membrane-spanning branches, as demonstrated by X-ray crystallography studies of photosystem I from cyanobacteria. Plastoglobules associated with the thylakoid membrane are believed to function as a reservoir for excess phylloquinone, and may also function in its metabolism. Edward A. Doisy and Henrik Dam received the Nobel Prize in Physiology or Medicine in 1943 for their discovery of vitamin K and its chemical structure. The structure of vitamin K is shown in Figure \(\PageIndex{25}\).
The menaquinones are related bacterial products, which function in the respiratory and photosynthetic electron transport chains of bacteria. They have a variable number (4 to 10) of isoprenoid units in the tail, and they are sometimes designated ‘MK-4’ to ‘MK-10’. In contrast to phylloquinone, these are usually highly unsaturated. In some species, there are methyl or other groups attached to the naphthoquinone moiety. Remarkably high concentrations of menaquinones are present in membranes of some extremophiles such as the haloarchaea, where it has been suggested that they act as ion permeability barriers and as a powerful shield against oxidative stress in addition to their functions as electron and proton transporters.
Phylloquinone is an essential component of the diet of animals and has been termed 'vitamin K1'. It must be supplied by green plant tissues, where it occurs in the range 400-700 μg/100 g, or seed oils. The menaquinones, the main source of which in the human diet is cheese and yogurt, also have vitamin K activity and are termed 'vitamin K2'. They account for about 10-25% of the vitamin K content of the Western diet. A synthetic saturated form of this, which is used in animal feeds, is known as 'vitamin K3 or menadione', though strictly speaking it is not a vitamin but a pro-vitamin in that it can be converted to the menaquinone MK-4 in animal tissues by addition of a phytyl unit; it is too toxic for human nutrition. Vitamin K forms are absorbed from the intestines and transported in plasma in the form of lipoproteins in a similar manner to the other fat-soluble vitamins. Different tissues have differ storage capacities and presumably requirements for the various forms of vitamin K. As a high proportion is excreted, there appears to be a requirement for a constant intake. Vitamin K1 is taken up rapidly by the liver, but vitamin K2 remains in the plasma for much longer and maybe the main source of the vitamin in peripheral tissues. In addition, it has been established that some dietary phylloquinone is converted to menaquinone-4 in animals, although the quantitative significance has still to be established; the mechanism involves conversion to menadione in the intestines followed by transport to tissues where a geranylgeranyl side-chain is attached by a specific prenyl transferase.
The primary role of vitamin K in animal tissues is to act as a cofactor specific to the vitamin K-dependent enzyme γ-glutamyl carboxylase in the endoplasmic reticulum in the liver mainly. Its function is the post-translational carboxylation of glutamate residues to form γ-carboxyglutamic acid in proteins, such as prothrombin. In this way, prothrombin and three related proteins are activated to promote blood clotting. The γ-carboxyglutamic acid residues are located at the binding site for Ca2+, and are vital for the activity of the enzyme. Phylloquinone must first be converted to the reduced form, phylloquinol, which is the actual cofactor for the enzyme; molecular oxygen and carbon dioxide are both required also. Phylloquinol donates hydrogen to the glutamic acid residue and is oxidized in the process to 2,3-epoxyphylloquinone. A further enzyme, vitamin K epoxide reductase, regenerates phylloquinone by reduction of the epoxide in a dithiol-dependent reaction so that this can be re-utilized many times ("the vitamin K cycle", shown below in Figure \(\PageIndex{26}\)).
Menaquinones undergo the same cycle of reaction. By interfering with the last step in the metabolic cycle, warfarin, the rodenticide, prevents blood clotting. In the same way, a deficiency in vitamin K results in the inhibition of blood clotting and can lead to brain hemorrhaging in malnourished newborn infants, though this is not seen in adult humans, presumably because intestinal bacteria produce sufficient for our needs. Vitamin K-dependent proteins are also known to have important functions in the central and peripheral nervous systems, and vitamin K influences sphingolipid biosynthesis in the brain.
Unsaturated isoprene units rather than phytol are used for the biosynthesis of menaquinones, and they differ from phylloquinone with respect to their chemical structure and pharmacokinetics. It is now apparent that vitamin K2 (MK-4 especially) has a number of different actions, some with specificities for particular tissues. For example, osteocalcin is a γ-carboxyglutamic acid-containing protein, which forms a strong complex with the mineral hydroxyapatite (calcium phosphate) of bone; it must be carboxylated to function properly and vitamin K2 appears to be of particular importance in this instance. Vitamin K2 also regulates bone remodeling by osteoclasts to remove old or damaged bone and its replacement by new bone. In addition, vitamin K2 is involved in vascular calcification, cell growth, and apoptosis. Side effects of the use of anticoagulants that bind to vitamin K can be osteoporosis and increased risk of vascular calcification. Although careful control of the dosage is necessary, vitamin K2 may be a useful adjunct for the treatment of osteoporosis, and it may reduce morbidity and mortality in cardiovascular health by reducing vascular calcification.
Excess vitamin K1 and the menaquinones are catabolized in the liver by a common degradative pathway in which the isoprenoid side chain is shortened to yield carboxylic acid aglycones such as menadiol, which can be excreted in bile and urine as glucuronides or sulfates.
Dolichols and Polyprenols
Polyisoprenoid alcohols, such as dolichols, are ubiquitous if minor components, relative to the glycerolipids, of membranes of most living organisms from bacteria to mammals. They are hydrophobic linear polymers, consisting of up to twenty isoprene residues or a hundred carbon atoms (or many more in plants especially), linked head-to-tail, with a hydroxy group at one end (α-residue) and a hydrogen atom at the other (ω-end). In dolichols (or dihydropolyprenols), the double bond in the α-residue is hydrogenated, and this distinguishes them from the polyprenols with a double bond in the α-residue. Their structures are shown in Figure \(\PageIndex{27}\).
Polyisoprenoid alcohols are further differentiated by the geometrical configuration of the double bonds into three subgroups, i.e., di-trans-poly-cis, tri-trans-poly-cis, and all-trans. For many years, it was assumed that polyprenols were only present in bacteria and plants, especially photosynthetic tissues, while dolichols were found in mammals or yeasts, but it is now known that dolichols can also occur at low levels in bacteria and plants, while polyprenols have been detected in animal cells. Solanesol is a related and distinctive plant product with trans double bonds only and is a precursor of plastoquinone.
Within a given species, components of one chain length may predominate, but other homologs are usually present. The chain length of the main polyisoprenoid alcohols varies from 11 isoprene units in eubacteria, to 16 or 17 in Drosophila, 15 and 16 in yeasts, 19 in hamsters, and 20 in pigs and humans. In plants, the range is from 8 to 22 units, but some species of plant have an additional class of polyprenols with up to 40 units. In tissues, polyisoprenoid alcohols can be present in the free form, esterified with acetate or fatty acids, phosphorylated or monoglycosylated phosphorylated (various forms), depending on species and tissue. Polyisoprenoid alcohols per se do not form bilayers in aqueous solution, but rather a type of lamellar structure. However, they are found in most membranes, especially the plasma membrane of liver cells and the chloroplasts of plants.
Dolichoic acids, i.e., related molecules with a terminal carboxyl group and containing 14–20 isoprene units, have been isolated from the substantia nigra of the human brain. However, they were barely detectable in the pig brain.
Biosynthesis of the basic building block of dolichols, e.g. isopentenyl diphosphate, follows either the mevalonate pathway or a more recently described methylerythritol phosphate pathway discussed in relation to cholesterol biosynthesis previously. Farnesyl pyrophosphate is the primary precursor in the biosynthesis of polyprenols and is the branch point in sterol/isoprene biosynthesis, depending on the nature of the organism (see a further note below). Subsequent formation of the linear prenyl chain is accomplished by cis-prenyl transferases that catalyze the condensation of isopentenyl pyrophosphate and farnesyl pyrophosphate and then the growing the allylic prenyl diphosphate chain. The end products are polyprenyl pyrophosphates, which are dephosphorylated first to polyprenol phosphate and thence to the free alcohol. Finally, a specific reductase has been identified from human tissues that catalyzes the reduction of the double bond in position 2 to produce dolichols. There is a family of cis-prenyl transferases, present in both eukaryotes and bacteria, that in addition to the synthesis of dolichols can catalyze the formation of isoprenoid carbon skeletons from neryl pyrophosphate (C10) to natural rubber (C>10,000), including the polyisoprenoid phosphates involved in protein glycosylation as discussed in the next section. The reactions in the synthesis of dolichol are shown in Figure \(\PageIndex{28}\).
Although polyprenols and dolichols were first considered to be simply secondary metabolites, they are now known to have important biological functions. Glycosylation of asparagine residues is the main protein modification in all three domains of life, and phosphorylated polyisoprenoids, including dolichols, are essential to this process (next section). There is also a suggestion that free dolichol may have a beneficial antioxidant function in cell membranes.
Polyisoprenoid Phosphates and Glycosylation of Proteins
Glycosylated phosphopolyisoprenoid alcohols are the carriers of oligosaccharide units for transfer to proteins and as glycosyl donors, i.e., they are substrates for glycosyl transferases for the biosynthesis of glycans in a similar manner to the cytosolic sugar nucleotides. They differ from the latter in their intracellular location, with the lipid portion in the membrane of the endoplasmic reticulum and the oligosaccharide portion specifically located either on the cytosolic or lumenal face of the membrane. The degree of unsaturation and chain length of the product is important for recognition by the enzymes in the next stage of the pathway.
Dolichol phosphates: In eukaryotes, N-glycosylation begins on the cytoplasmic side of the endoplasmic reticulum with the transfer of carbohydrate moieties from nucleotide-activated sugar donors, such as uridine diphosphate N-acetylglucosamine, onto dolichol phosphate. Then, N-acetylglucosamine phosphate is added to give dolichol-pyrophosphate linked to N-acetylglucosamine, to which a further N-acetylglucosamine unit is added followed by five mannose units, the last catalyzed by dolichol phosphate mannose synthase, which is also essential for GPI-anchor biosynthesis. The dolichol-PP glycan is shown in Figure \(\PageIndex{29}\).
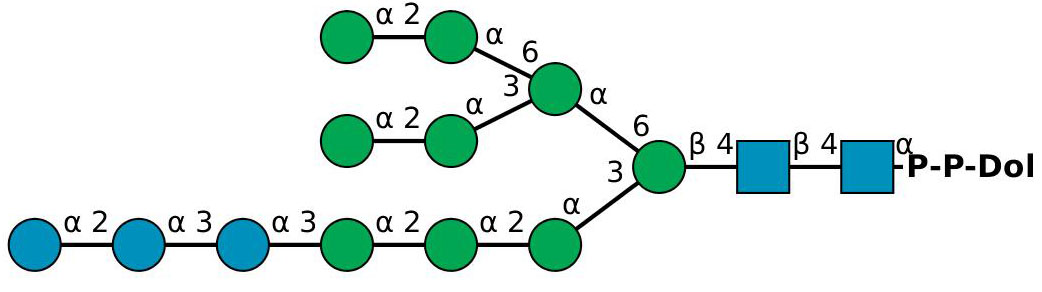
The resulting dolichol-pyrophosphate heptasaccharide is then flipped across the endoplasmic reticulum membrane to the luminal face with the aid of a “flippase”. Four further mannose and three glucose residues are added to the oligosaccharide chain by means of glycosyltransferases, which utilize as donors dolichol-phospho-mannose and dolichol-phospho-glucose, which are also synthesized on the cytosolic face of the membrane and flipped across to the luminal face. In humans, the final lipid product is a C95-dolichol pyrophosphate-linked tetradecasaccharide, the oligosaccharide unit of which is transferred from the dolichol carrier onto specific asparagine residues on a developing polypeptide in the membrane. The carrier dolichol-pyrophosphate is dephosphorylated to dolichol-phosphate then diffuses or is flipped back across the endoplasmic reticulum to the cytoplasmic face.
The Archaea use dolichol in their synthesis of lipid-linked oligosaccharide donors with both dolichol phosphate (Euryarchaeota) and pyrophosphate (Crenarchaeota) as carriers; these can have variable numbers of isoprene units many of which can be saturated. In the haloarchaeon Haloferax volcanii, for example, a series of C55 and C60 dolichol phosphates with saturated isoprene subunits at the α- and ω-positions is involved in the glycosylation reaction of target proteins, while similar lipid carriers of oligosaccharide units appear to be present in methanogens. Archaea of course use isoprenyl ethers linked to glycerol as major membrane lipid components in addition to unusual carotenoids such as the C50 bacterioruberins. In many of these species, isoprenoid biosynthesis is via the 'classical' mevalonate pathway, but in other species, some aspects of this pathway differ.
Undecaprenylphosphate: Most other bacteria use undecaprenyl-diphosphate-oligosaccharide as a glycosylation agent in a similar way for the biosynthesis of peptidoglycan, the main component of most bacterial cell walls and a structure unique to bacteria, of many other cell-wall polysaccharides, including lipopolysaccharides, O-antigenic polysaccharides and capsular polysaccharides, and of N-linked protein glycosylation in both in Gram-negative and Gram-positive bacteria. Undecaprenyl phosphate (a C55 isoprenoid), also referred to as bactoprenol, is the essential lipid intermediate. Its structure is shown in Figure \(\PageIndex{30}\).
It differs from the dolichol phosphates mainly in that the terminal unit is unsaturated, and is synthesized by the addition of eight units of isopentenyl pyrophosphate to farnesyl pyrophosphate, a reaction catalyzed by undecaprenyl pyrophosphate synthase in the cytoplasm, followed by the removal of a phosphate group. Undecaprenyl phosphate is required for the synthesis and transport of glycans for external polymer formation. Thus, glycans are covalently transferred to the carrier lipid by membrane-embedded or membrane-associated enzymes using nucleotide-activated precursors. For example, the carrier lipid with GlcNAc-MurNAc-peptide monomers, i.e., as lipid II, is hydrophilic and is then transported across the cytoplasmic membrane to external sites for peptidoglycan formation. Similarly, it is required for the synthesis of lipoteichoic acids and lipopolysaccharide O-antigens.
Lipid II: Undecaprenyl diphosphate-MurNAc-pentapeptide-GlcNAc, often simply termed lipid II, is the last significant lipid intermediate in the construction of the peptidoglycan cell wall in bacteria (Lipid I is the biosynthetic precursor lacking the N-acetylgluosamine residue). Its structure is shown below in Figure \(\PageIndex{31}\).
Figure \(\PageIndex{31}\): Lipid II
This molecule must be translocated from the cytosolic to the exterior membrane of the organism, and three different protein classes have been identified that can accomplish this of which ATP-binding cassette (ABC) transporters are best characterized. Once across the membrane, lipid II is cleaved to provide the MurNAc-pentapeptide-GlcNAc monomer, which undergoes polymerization and crosslinking to form the complex peptidoglycan polymer that provides strength and shape to bacteria. The undecaprenyl-pyrophosphate remaining is hydrolyzed to undecaprenyl phosphate by a membrane-integrated member of the type II phosphatidic acid phosphatase family and is recycled back to the interior of the membrane by an as yet unidentified transport mechanism. The turnover rate is very high so the lipid II cycle is considered to be the rate-limiting step in peptidoglycan biosynthesis. There is also some evidence that lipid II has a function on the inner leaflet of the cytoplasmic membrane in organizing the proteins of the cytoskeleton. Because of its highly conserved structure and accessibility on the surface membrane, the proteins involved in the synthesis and transport of lipid II are considered important targets for the development of novel antibiotics. Figure \(\PageIndex{xx}\):
In a few prokaryotes, the membrane intermediate has a polyprenyl-monophosphate-glycan structure instead of lipid II, and undecaprenyl-phosphate-L-4-amino-4-deoxyarabinose is involved in lipid A modification in Gram-negative bacteria, for example. There are obvious parallels with the involvement of glycosylated phosphopolyisoprenoid alcohols as carriers of oligosaccharide units for transfer to proteins and as glycosyl donors in higher organisms (see above).
β‑D‑Mannosyl phosphomycoketide is an isoprenoid phosphoglycolipid found in the cell walls of Mycobacterium tuberculosis, the lipid component of which is a C32-mycoketide, consisting of a saturated oligoisoprenoid chain with five chiral methyl branches. It acts as a potent antigen to activate T-cells upon presentation by CD1c protein. Its structure is shown in Figure \(\PageIndex{32}\).
Farnesyl Pyrophosphate and Related Compounds
Farnesyl pyrophosphate is a key intermediate in the biosynthesis of sterols such as cholesterol, and it is the donor of the farnesyl group in the biosynthesis of dolichols and polyprenols (see above) as well as for the isoprenylation of many proteins (see the web page on proteolipids). However, it is also known to mediate various biological reactions in its own right via interaction with a specific receptor. It is synthesized by two successive phosphorylation reactions of farnesol.
Presqualene diphosphate is unique among the isoprenoid phosphates in that it contains a cyclopropylcarbinyl ring. In addition to being a biosynthetic precursor of squalene, and thence of cholesterol, it is a natural anti-inflammatory agent, which functions by inhibiting the activity of phospholipase D and the generation of superoxide anions in neutrophils.
Their structures are shown in Figure \(\PageIndex{32}\).




.png?revision=1&size=bestfit&width=283&height=253)