21.4: Biosynthesis of Membrane Sphingolipids
- Page ID
- 81290
by William (Bill) W. Christie and Henry Jakubowski.
This section is an abbreviated and modified version of material from the Lipid Web, an introduction to the chemistry and biochemistry of individual lipid classes, written by William Christie.
Sphingolipids
Introduction:
The sphingolipids comprise a wide range of complex lipids in which the defining component is a long-chain or sphingoid base, which in living tissues is usually linked to a fatty acid via an amide bond. J.L.W. Thudichum, a German chemist working in London, first coined the root term “sphingo-” in 1884 following his discovery of the first glycosphingolipids, because the enigmatic nature of the molecules reminded him of the riddle of the sphinx. Regretfully, the importance of his work was not recognized until 25 years after his death, and it was 1947 before the term “sphingolipide” was introduced by Herbert Carter and colleagues. While they are much less enigmatic than they once were, sphingolipids are extremely versatile molecules that continue to fascinate as new knowledge is gained of their functions in healthy (and diseased) animal and plant tissues. They are found in only a few bacterial genera, but they are present in Sphingomonas, Sphingobacterium and a few other species, and many pathogenic species utilize host sphingolipids to promote infections. Novel sphingolipid structures continue to be reported, and as an example at the last count, 188 of the complex sphingolipids classified as gangliosides, with variations in the complex carbohydrate component alone, had been characterized in vertebrates.
Long-chain or sphingoid bases, of which sphingosine is typical, are the basic elements and are the simplest possible functional sphingolipids. They vary in chain length and in the presence of various functional groups including double bonds of both the cis- and trans-configuration at different locations in the aliphatic chain. Ceramides, which contain sphingoid bases linked to fatty acids by amide bonds, vary appreciably in the compositions of both aliphatic components, depending on their biological origins. The structure of sphingosine and ceramide, the sphingolipid building blocks, are shown in Figure \(\PageIndex{1}\).
Long-chain bases and ceramides have important biological properties in their own right, for example in relation to intra- and inter-cellular molecular signaling, especially in animal cells, while another relatively simple sphingolipid, sphingosine-1-phosphate, is now recognized as a key factor in countless aspects of animal metabolism. The concentrations of these bioactive lipids respond rapidly to the action of specific stimuli and then regulate downstream effectors and targets.
Ceramides are the precursors of a multitude of sphingo-phospho- and sphingo-glycolipids with an immense range of functions in tissues. The properties and functions of these complex sphingolipids are quite distinct from those of the comparable glycerophospho- and glyceroglycolipids. For example in animals, sphingomyelin has structural similarities to phosphatidylcholine, but has very different physical and biological properties, while the complex oligoglycosylceramides and gangliosides(glycosphingolipids, of which glucosylceramide is the precursor, have no true parallels among the glyceroglycolipids. Figure \(\PageIndex{2}\) shows the structure of the complex sphingolipids sphingomyelin and glucosylceramide.
Complex sphingolipids are synthesized in the endoplasmic reticulum and Golgi, but are located mainly in the plasma membrane of most mammalian cells where they have a structural function and also serve as adhesion sites for proteins from the extracellular tissue. The glycosphingolipids are especially important for myelin formation in the brain. However, sphingolipids have intracellular functions in all cellular compartments, including the nucleus. The first five carbon atoms of the sphingoid base in sphingolipids have a highly specific stereochemistry and constitute a key feature that has been termed the ‘sphingoid motif’, which in comparison to other lipid species facilitates a relatively large number of noncovalent interactions with other membrane lipids, via hydrogen-bonding, ion-ion interactions and induced dipole-induced dipole interactions. A distinctive property of sphingolipids in membranes is that they spontaneously form transient nanodomains termed 'rafts', usually in conjunction with cholesterol, where such proteins as enzymes and receptors congregate to carry out their signaling and other functions. Thus, in addition to their direct effects on metabolism, sphingolipids affect innumerable aspects of biochemistry indirectly via their physical properties.
While it may be obvious that a well-balanced sphingolipid metabolism is important for health in animals, increasing evidence has been acquired to demonstrate that impaired sphingolipid metabolism and function are involved in the pathophysiology of many of the more common human diseases. These include diabetes, various cancers, microbial infections, Alzheimer's disease and other neurological syndromes, and diseases of the cardiovascular and respiratory systems. In humans, a number of important genetic defects in sphingolipid metabolism or sphingolipidoses have been detected, especially storage diseases associated with the lysosomal compartment where sphingolipids are catabolized. Sphingolipids and their metabolism are therefore likely to prove of ever increasing interest to scientists.
There are appreciable differences in sphingolipid compositions and metabolism between animal and plant cells, both with respect to the aliphatic components and especially the polar head groups, although there are also some important similarities. While sphingomyelin is the most abundant sphingolipid in animals, it does not occur in plants and fungi. Although less is known of the role they play in plants, it has become apparent that complex sphingolipids are much more abundant in plant membranes than was once believed, and it is now recognized that they are key components of the plasma membrane and endomembrane system.
Some General Comments on Sphingolipid Metabolism
The biosynthesis and catabolism of sphingolipids involves a large number of intermediate metabolites, all of which have distinctive biological activities of their own. In animals, the relationships between these metabolites have been rationalized in terms of a ‘sphingomyelin, sphingolipid or ceramide cycle’, as shown in Figure \(\PageIndex{3}\).
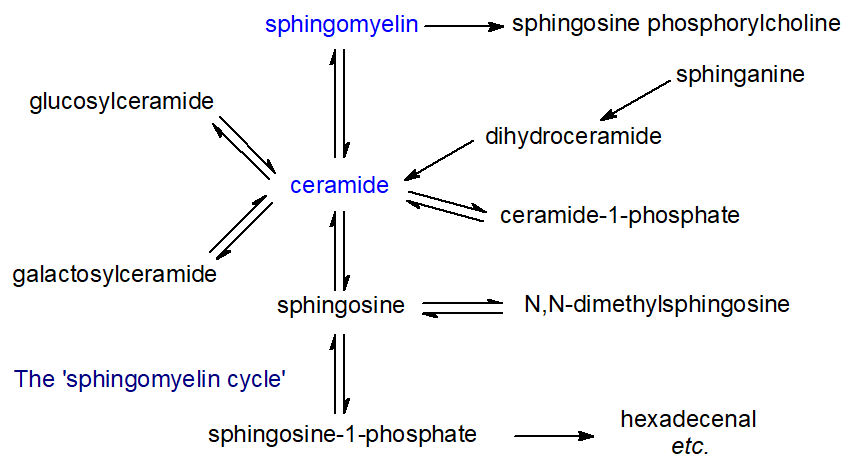
Many different enzymes (and their isoforms) are involved, and their activities depend on a number of factors, including intracellular locations and mechanisms of activation. Each of the various compounds in these pathways has characteristic metabolic properties. Thus, free sphingosine and other long-chain bases, which are the primary precursors of ceramides and thence of all the complex sphingolipids, function as mediators of many cellular events, for example by inhibiting the important enzyme protein kinase C. Ceramides are involved in cellular signaling, and especially in the regulation of apoptosis, and cell differentiation, transformation and proliferation, and most stress conditions. In contrast, sphingosine-1-phosphate and ceramide-1-phosphate promote cellular division (mitosis) as opposed to apoptosis, so that the balance between these lipids and ceramide and/or sphingosine levels in cells is critical and necessitates exquisite control in each cellular compartment.
Similarly, the ‘structural’ sphingolipids, such as sphingomyelin, monoglycosylceramides, oligoglycosylceramides and gangliosides, all have unique and characteristic biological functions, some of which are due to their physical properties and location within rafts, nanodomains of membranes. Most of the reactions in the sphingomyelin cycle are reversible and the relevant enzymes are located in the endoplasmic reticulum, Golgi, plasma membrane, and mitochondria, but the more complex sphingolipids are catabolized in the lysosomal compartment. Sphingolipids are especially important in providing the permeability barrier in the skin, where they are characterized by the presence of ultra-long fatty acyl components as well as fatty acyl groups linked to a hydroxyl group at the terminal end of the N‑linked fatty acids (thereby generating a three‑chain rather than a two‑chain molecule).
Metabolic pathways that are comparable to those of the sphingomyelin cycle are believed to occur in plants, as shown in Figure \(\PageIndex{4}\), although they have not been studied as extensively as those in animals.
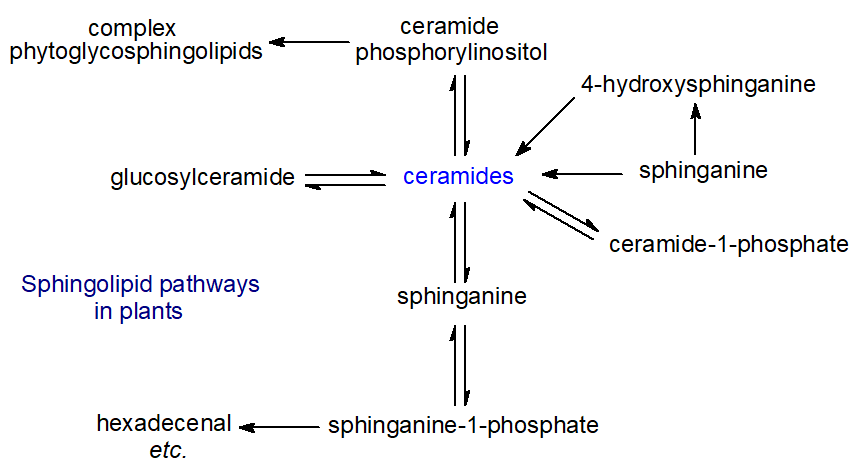
However, sphingolipid metabolites such as sphingosine-1-phosphate (or analogues) have been linked to programmed cell death, signal transduction, membrane stability, host-pathogen interactions and stress responses, for example. Plants also have a unique range of complex sphingolipids in their membranes, such as ceramide phosphorylinositol and the phytoglycosphingolipids, and these are now known to constitute a higher proportion of the total lipids than had hitherto been supposed, although their functions have hardly been explored. While sphingolipids are produced by relatively few bacterial species, sulfono-analogues of long-chain bases and ceramides (capnoids) are produced by some specie.
Fatty acid Components of Sphingolipids
The fatty acids of sphingolipids are very different from those of glycerolipids, consisting of very-long-chain (up to C26) odd- and even-numbered saturated or monoenoic and related 2(R)-hydroxy components, while even longer fatty acids (C28 to C36) occur in spermatozoa and the epidermis. The dienoic acid 15,18‑tetracosadienoate (24:2(n‑6)), derived from elongation of linoleic acid, is found in the ceramides and other sphingolipids of a number of different tissues, but at relatively low levels. Polyunsaturated fatty acids are only rarely present, although sphingomyelins of testes and spermatozoa are exceptions in that they contain such fatty acids, which are even longer in chain-length (up to 34 carbon atoms) and include 28:4(n‑6) and 30:5(n‑6). Skin ceramides also contain unusual very-long-chain fatty acids, while yeast sphingolipids are distinctive in containing mainly C26 fatty acids. In plants and yeasts, a similar range of chain-lengths occur as in animals, but 2-hydroxy acids predominate sometimes accompanied by small amounts of 2,3‑dihydroxy acids; saturated fatty acids are most abundant, but monoenes are present in higher proportions in the Brassica family (including Arabidopsis) and a few other species. Some fungal species contain monoenoic fatty acids with a trans-3 double bond and/or a hydroxyl group. Figure \(\PageIndex{5}\) shows typical sphingolipid fatty acids.
Very-long-chain saturated and monoenoic fatty acids for sphingolipid biosynthesis are produced from medium-chain precursors by elongases (ELOVL) in the endoplasmic reticulum of cells in mammals, and there is increasing evidence that specific isoforms are involved in the biosynthesis of certain ceramides. For example, ELOVL1 has been linked to the production of ceramides with C24 fatty acids (saturated and unsaturated), while ELOVL4 is responsible for the ultra-long-chain fatty acids in skin. Yeasts possess three elongation enzymes: Elo1 (for medium to long-chain fatty acids), Elo2 (up to C22) and Elo3 (up to C26).
The hydroxyl group is believed to add to the hydrogen-bonding capacity of the sphingolipids, and it helps to stabilize membrane structures and strengthen the interactions with membrane proteins. Hydroxylation is effected by a fatty acid 2-hydroxylase in mammals, i.e. an NAD(P)H-dependent monooxygenase, which is an integral membrane protein of the endoplasmic reticulum. It converts unesterified long-chain fatty acids to 2‑hydroxy acids in vitro and probably also in vivo. For example, experimental evidence has been obtained that is consistent with 2‑hydroxylation occurring at the fatty acid level prior to incorporation into ceramides in the brain of mice where the enzyme is expressed at high levels. A second enzyme of this kind is known to exist but has yet to be characterized, and it is possible that a proportion of the odd-chain fatty acids in brain are synthesized by Peroxisomal α-oxidation of the 2‑hydroxy acids. Similarly, in skin, 2‑hydroxy and non-hydroxy fatty acids as their CoA esters are used with equal facility for ceramide biosynthesis by ceramide synthases. As mutations in the fatty acid 2‑hydroxylase in humans and mice give rise to demyelination disorders, such as leukodystrophy, it is evident that sphingolipids containing 2‑hydroxy acids have unique functions in membranes that cannot be substituted by non-hydroxy analogues.
In plants, it appears that 2‑hydroxyl groups are inserted into fatty acyl chains while they are linked to ceramide, as ceramide synthase does not accept hydroxy fatty acids in vitro at least. Two fatty acid 2‑hydroxylases (di-iron-oxo enzymes) have been found in Arabidopsis, with one specific for very-long-chain fatty acids and one for palmitic acid. In fungi, a hydroxyl group is inserted at C2 of the fatty acid in a dihydroceramide intermediate.
Although the fatty acids are only occasionally considered in terms of the biological functions of sphingolipids, their influence is considerable, especially but not only in relation to their physical properties and function in membranes. For example, very-long-chain fatty acids may play a role in stabilizing highly curved membrane domains as is required during cell division. The hydrophobic nature of the fatty acyl groups (together with the long-chain bases) enables the hydrogen bonding that is essential for the formation of raft nanodomains in membranes. As a general rule, lipid bilayers containing sphingolipids with 2-hydroxy-fatty acyl or 4-hydroxy-sphingoid base moieties, tend to generate condensed and more stable gel phases with higher melting temperatures than their non-hydroxylated equivalents, because they have a more extended and strengthened intermolecular hydrogen bonding network. Changes in fatty acid composition are seen in some disease states, and for example increased concentrations of fatty acids >C24 are a feature of adrenoleukodystrophy, an X-linked genetic disorder.
Removal of very-long-chain fatty acids from sphingolipids in mutants of the model plant Arabidopsis inhibits completely the development of seedlings. As example of a more specific interaction, it has been demonstrated that synthetic glycerolipids must contain very-long-chain fatty acids (C26) to allow growth in yeast mutants lacking sphingolipids, probably by stabilizing the proton-pumping enzyme H+-ATPase. Similarly, ceramides containing different fatty acids can be used in highly specific ways. Thus in fungi, C16 or C18 hydroxy acids are used exclusively for synthesis of glucosylceramide, while those containing very-long-chain C24 and C26 hydroxy acids are used only for synthesis of glycosyl inositol phosphorylceramide anchors for proteins. In plants, sphingolipids containing 2-hydroxy acids are protective against oxidative and other biotic stresses.
Links between Glycerolipid and Sphingolipid Metabolism
Sphingolipid metabolism and glycerolipid metabolism have been widely treated as separate sciences until relatively recently, partly for historical reasons and partly because the analysis of the two lipid groups required different approaches and skills. However, there are many areas where the two overlap, not least because phosphatidylcholine is the biosynthetic precursor of sphingomyelin in animal cells, while in plants and fungi, phosphatidylinositol is the biosynthetic precursor of ceramide phosphorylinositol. In contrast, ethanolamine phosphate derived from the catabolism of sphingolipids via sphingosine 1-phosphate is recycled for the biosynthesis of phosphatidylethanolamine, and this is essential for survival in the protozoan parasite Trypanosoma brucei. In studies in vitro, sphingosine 1-phosphate has been shown to be an activator of the phospholipase C involved in the hydrolysis of the lipid mediator phosphatidylinositol 4,5-bisphosphate with formation of diacylglycerols and inositol triphosphate. The location and functions of glycerophospholipids in membranes is influenced both positively and negatively by sphingolipid-rich domains or rafts in membranes.
In addition, there are several examples of phosphoinositides and other complex lipids binding to enzymes of sphingolipid metabolism, either as part of a regulatory function that controls their activity or to facilitate their location to various membranes. Thus, sphingosine kinase 2, one of the enzymes responsible for the biosynthesis of sphingosine 1-phosphate, binds to phosphatidylinositol monophosphates, while the ceramide kinase responsible for the biosynthesis of ceramide 1-phosphate requires phosphatidylinositol 4,5-bisphosphate to function. Similarly, the CERT protein involved in ceramide transport has a binding site for phosphatidylinositol 4-phosphate. Sphingomyelin production at the trans-Golgi network triggers a signaling pathway leading to dephosphorylation of phosphatidylinositol 4-phosphate, interrupting transport of cholesterol and sphingomyelin. Again, the interactions are not solely in one direction as ceramide 1‑phosphate (with phosphatidylinositol 4,5-bisphosphate) binds to the specific phospholipase A2 (cPLA2α) responsible for the hydrolysis of phosphatidylinositol and thence the release arachidonic acid for eicosanoid production. Other than the phosphoinositides, phosphatidylserine activates the neutral sphingomyelinase in brain.
Long-Chain (Sphingoid) Bases
Long-chain/sphingoid bases are the characteristic and defining structural unit of the sphingolipids, which are important structural and signaling lipids of animals and plants and of a few bacterial species. These are long-chain aliphatic amines, containing two or three hydroxyl groups, and often a distinctive trans-double bond in position 4. To be more precise, they are 2-amino-1,3-dihydroxy-alkanes or alkenes with (2S,3R)‑erythro stereochemistry, often with various further structural modifications in the alkyl chain. They are important for the physical and biological properties of all of the more complex sphingolipids, but free sphingoid bases are also bioactive and interact with specific receptors and target molecules. As discussed below, the mechanisms for biosynthesis of sphingoid bases and of the N-acylated form (ceramides) are intimately linked.
Structures and Occurrence
In animal tissues, the most common or abundant of the sphingoid bases is sphingosine ((2S,3R,4E)-2-amino-4-octadecene-1,3-diol) or sphing-4E-enine, i.e., with a C18 aliphatic chain, hydroxyl groups in positions 1 and 3 and an amine group in position 2; the double bond in position 4 has the trans (or E) configuration. This was first characterized in 1947 by Professor Herbert Carter, who was also the first to propose the term “sphingolipides” for those lipids containing sphingosine. It is usually accompanied by the saturated analogue dihydrosphingosine (or sphinganine). Sphingoid bases are illustrated in Figure \(\PageIndex{6}\).
For shorthand purposes, a nomenclature similar to that for fatty acids can be used; the chain length and number of double bonds are denoted in the same manner with the prefix 'd' or 't' to designate di- and trihydroxy bases, respectively. Thus, sphingosine is denoted as d18:1 and phytosphingosine is t18:0. The position of the double bond may be indicated by a superscript, i.e., 4-sphingenine is d18:1Δ4t or 4E-d18:1. While alternative nomenclatures are occasionally seen in publications, they are not recommended.
The number of different long-chain bases that has been found in animals, plants and microorganisms now amounts to over one hundred, and many of these may occur in a single tissue or organism, but almost always as part of a complex lipid with an N-acyl-linked fatty acid and often phosphate or carbohydrate functional groups, as opposed to in the free form. The aliphatic chains can contain from 14 to as many as 28 carbon atoms, and most often they are saturated, monounsaturated or diunsaturated, with double bonds of either the cis or trans configuration. For example, the main dienoic long-chain base (sphingadienine) in human plasma is D-erythro-1,3-dihydroxy-2-amino-4-trans,14-cis-octadecadiene, and this is especially abundant in kidney, with more in women than in men. It is not present in zebra fish, widely used as a model species. Forms with three double bonds, such as sphinga-4E,8E,10E-trienine, sometimes with a methyl group in position 9, have been found the sphingolipids of some marine invertebrates and in a dinoflagellate. In addition, long-chain bases can have branched chains with methyl substituents in the omega‑1 (iso), omega‑2 (anteiso) or other positions, hydroxyl groups in positions 4, 5 or 6, ethoxy groups in position 3, and even a cyclopropane ring in the aliphatic chain in some organisms. N-Methyl, N,N-dimethyl and N,N,N-trimethyl derivatives of sphingoid bases have been detected in mouse brain.
The main C18 components of long-chain bases of sphingomyelins of some animal tissues are accompanied by small amounts of C16 to C19 dihydroxy bases, although the latter attain higher proportions in tissues of ruminant animals. In gangliosides from human brain and intestinal tissues, eicosasphingosine (2S,3R,4E-d20:1) occurs in appreciable concentrations with variable amounts in different regions and membranes. However, human skin contains an especially wide range of isomers, including saturated, monoenoic and 6-hydroxy bases and phytosphingosines from C16 to C28 in chain-length. Shorter-chain bases are found in many insect species, and in the fruit fly, Drosophila melanogaster, which is widely used as a model species in genetic and metabolic experiments, the main components are C14 bases. In contrast to higher animals, nematodes such as Caenorhabditis elegans produce C17 iso-methyl-branched sphingoid bases, which are essential for normal sphingolipid function in the organism.
The long-chain base composition of individual lipids can vary markedly between species, tissues, organelles and even different membranes within a single organelle. For example, the data in Table \(\PageIndex{1}\) is perhaps from an extreme example, but it illustrates that remarkable differences that can exist among lipids in one cellular component (rat liver mitochondria). Only part of the data from the paper cited is listed, but it illustrates that 3-keto-sphinganine, produced in the first step of sphingosine biosynthesis (see below) and normally a minor component of sphingolipids - often not detectable, can vary from 28 to 100% of the sphingoid bases depending on the lipid class and membrane within the organelle.
| Table \(\PageIndex{1}\): Long chain base composition of some lipid components of mitochondria from rat liver. | ||||
| Type | Base (%) | |||
|---|---|---|---|---|
| d18:1 | d18:0-3keto | t21:1 (phyto) | Unidentified | |
| Ceramidesa | 18 | 28 | 53 | - |
| Glucosylceramidesa | 3 | 95 | - | 3 |
| Lactosylceramidesb | 100 | |||
| a whole mitochondria; b mitochondrial inner membrane Data from Ardail, D. et al. FEBS Letts, 488, 160-164 (2001). |
||||
Phytosphingosine or 4D-hydroxy-sphinganine ((2S,3R,4R)-2-amino-octadecanetriol) is a common long-chain base of mainly plant origin. It is a saturated C18-trihydroxy compound, although unsaturated analogues, for example with a trans (or occasionally a cis (Z)) double bond in position 8, i.e., dehydrophytosphingosine or 4D‑hydroxy-8-sphingenine, tend to be much more abundant. In many plant species, there are lipid class preferences also, and dihydroxy long-chain bases are more enriched in glucosylceramides than in glycosylinositolphosphoceramides, for example. This is true in the model plant Arabidopsis thaliana, where the data listed for whole tissue is probably representative largely of the latter lipid, as shown in Table \(\PageIndex{2}\) below.
| Table \(\PageIndex{2}\): Sphingolipid long-chain base composition of whole tissue and glucosylceramides from Arabidopsis thaliana. | ||||||
| Base (%) | ||||||
|---|---|---|---|---|---|---|
| t18:1 (8Z) | t18:1 (8E) | t18:0 | d18:1 (8Z) | d18:1 (8E) | d18:0 | |
| Whole tissue | 12 | 70 | 13 | 4 | 1 | |
| Glucosylceramides | 44 | 22 | 5 | 28 | 2 | |
| Data from Sperling, P. et al. Plant Physiol. Biochem., 43, 1032-1038 (2005) | ||||||
Other plant long-chain bases have double bonds in position 4, which can be of either the cis or trans configuration, although trans-isomers are by far the more common, while the base d18:2Δ4E,8Z/E is relatively abundant in most plant species. In A. thaliana and related species, Δ4 long-chain bases are found mainly in the flowers and pollen and then exclusively as a component of the glucosylceramides. In general outwith Brassica species, the composition is dependent on species, but typically it is composed of up to eight different C18-sphingoid bases, with variable geometry of the double bond in position 8, i.e., (E/Z)-sphing-8-enine (d18:1Δ8), (4E,8E/Z)-sphinga-4,8-dienine (d18:2Δ4,8) and (8E/Z)-4-hydroxy-8-sphingenine (t18:1Δ8); d18:1Δ4, d18:0 and t18:0 tend to be present in small amounts only.
Phytosphingosine is not restricted to plants, but is found in significant amounts in intestinal cells and skin of animals, with much smaller relative proportions in kidney. Although non-mammalian sphingoid bases in general tend to be poorly absorbed from the intestines, a small proportion of the phytosphingosine and related sphingoid bases found in animal tissues may enter via the food chain.
Yeasts and fungi tend to have distinctive and characteristic long-chain base compositions. For example, filamentous fungi have 9-methyl-4E,8E-sphingadienine as the main sphingoid base in the glucosylceramides, as shown in Figure \(\PageIndex{7}\), but not in the ceramide phosphoinositol glycosides, while yeasts contain mainly the saturated C18 bases sphinganine and phytosphingosine, although some trans-4/8-unsaturated forms are usually present. Only a few bacterial species synthesize sphingolipids, but the family Bacteroidetes, which is abundant in the human gut is an important exception; they usually contain saturated (and branched) long-chain bases. Other pathogenic bacteria may utilize sphingolipids and sphingoid bases from their hosts.
Sphingoid bases are surface-active amphiphiles with critical micellar concentrations of about 20 μM in aqueous solutions; they probably exist in the gel phase at physiological temperatures. In that they bear a small positive charge at neutral pH, they are unusual amongst lipids, although their pKa (9.1) is lower than in simple amines as a consequence of intra-molecular hydrogen bonding. Together with their relatively high solubility (> 1μM), this enables them to cross membranes or move between membranes with relative ease. In so doing, they increase the permeability of membranes to small solutes. In esterified form in complex lipids, they participate in the formation of ordered lipid domains in membranes such as rafts.
In the complex sphingolipids, the sphingoid base is linked via the amine group to a fatty acid, including very-long-chain saturated or monoenoic and 2-hydroxy components, i.e., to form ceramides, which can be attached a polar head group, such as phosphate or a carbohydrate, via the primary hydroxyl moiety. An important exception is sphingosine-1-phosphate, which is not acylated and has signaling functions in cells akin to those of lysophospholipids.
Biosynthesis and Metabolism
Sphinganine biosynthesis
The basic mechanism for the biosynthesis of sphinganine involves condensation of palmitoyl-coenzyme A with L-serine, catalyzed by the membrane-bound enzyme serine palmitoyltransferase, requiring pyridoxal 5’-phosphate as a cofactor, which binds to a specific lysine residue on the enzyme. The reaction occurs on the cytosolic side of the endoplasmic reticulum in animal, plant and yeast cells with formation of 3-keto-sphinganine as illustrated in Figure \(\PageIndex{8}\).
This is believed to be the key regulatory or rate-limiting step in sphingolipid biosynthesis and is conserved in all organisms studied to date. Elimination of this enzyme is embryonically fatal in mammals and fruit flies. In mammals, serine palmitoyltransferase is a heterotrimer composed of two main subunits, designated SPTLC1 with either SPTLC2 or SPTLC3 (sometimes termed SPTLC2a and SPTLC2b, respectively). SPTLC1 is essential for activity, and it is ubiquitously expressed as is SPTLC2, while SPTLC3 is present in a relatively limited range of tissues and is most abundant in skin and placental tissue. In addition, there are two small subunits ssSPTA and ssSPTB (again other nomenclatures exist), which differ in a single amino acid residue, and may have regulatory functions; the active site is at the interface between the two main subunits. ssSPTA is essential for serine palmitoyltransferase function during development and hematopoiesis.
A possible mechanism for the 1st step in the pathway, catalyzed by serine palmitotyltransferase, is shown in Figure \(\PageIndex{9}\).
The addition of either of the two small subunits to the complexes changes the substrate preferences substantially and enables the synthesis of the wide range of homologs found in nature. In mammals, the SPTLC1-SPTLC2 complex forms C18 sphingoid bases specifically (with some C19, and C20), while the combination of SPTLC1 and SPTLC3 gives a broader product spectrum, including an anteiso-methylbranched-C18 isomer (from anteiso-methyl-palmitate as the precursor). Such branched bases are synthesized to a limited extent in human skin, but they are the main forms in lower invertebrates such as C. elegans. The activity of the serine palmitoyltransferase is governed by negative feedback and partly by orosomucoid (ORM-like or ORMDL) proteins, three in mammals (ORMDL1 to 3) and two in yeast (Orm1/2), which are ubiquitously expressed trans-membrane proteins located in the endoplasmic reticulum. The availability of serine is also an important factor.
Figure \(\PageIndex{10}\) shows an interactive iCn3D model of the human serine palmitoyltransferase complex (7K0M).
Figure \(\PageIndex{10}\): Human serine palmitoyltransferase complex (7K0M).. Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...tuGThTq9Vi4J58
- Gray and Plum (SPT1 A and E Chains
- Light Blue and Blue (SPT2 B and F Chains)
- Light Brown (small subunit A - ssA, C and G chains)
- Yellow (ORM D and H)
The second step in sphinganine biosynthesis is reduction of the keto group to a hydroxyl in an NADPH-dependent manner by a specific 3‑ketodihydrosphingosine reductase ('3KSR'), also on the cytosolic side of the endoplasmic reticulum, a step that must occur rapidly as the intermediate is rarely encountered in tissues. The enzymes are presumed to be in similar subcellular locations in plant cells.
In plants, serine palmitoyltransferase is a heterodimer composed of LCB1 and LCB2 subunits with some homology to the mammalian enzymes, while in the yeast Saccharomyces cerevisiae, there are three subunits: Lcb1, Lcb2, and Tsc3. In the few bacteria that synthesize sphingoid bases, serine palmitoyltransferase is a water-soluble homodimer. The enzyme in the apicomplexan parasite Toxoplasma gondii is a homodimer also in contrast to other eukaryotes, but it is located in the endoplasmic reticulum.
Free sphinganine formed in this way is rapidly N-acylated by acyl-coA to form dihydroceramides by dihydroceramide synthases, which in animals are located primarily on the endoplasmic reticulum, presumably on the cytoplasmic surface. Animals and plants have multiple isoforms of this enzyme, for which the abbreviated term ‘ceramide synthase’ is now widely applied as they utilize most other sphingoid bases, such as those produced by hydrolysis of sphingolipids, as substrates. They are unique gene products with each located on a different chromosome and with considerable variation in the expression of the enzymes in different cell types within each tissue. Each isoenzyme has distinct specificities for the chain-length of the fatty acyl-CoA moieties but to a limited extent only for the base, suggesting that ceramides containing different fatty acids have differing roles in cellular physiology. All of these enzymes have six membrane spanning regions, but the only substantial difference is in an 11-residue sequence in a loop between the last two putative transmembrane domains. Ceramides are central to all elements of sphingolipid biochemistry. These steps are illustrated in Figure \(\PageIndex{11}\).
Humans and mice have six ceramide synthases, which utilize subsets of acyl-CoAs and thus producing ceramides with specific acyl chain lengths. Of these, ceramide synthase 2 is most abundant and is specific for coA esters of very-long-chain fatty acids (C20 to C26); it is most active in lung, liver and kidney. Ceramide synthase 1 is specific for 18:0 and is located mainly in brain with lower levels in skeletal muscle and testes. Ceramide synthase 3 is responsible for the unusual ceramides of skin and testes and uses C26-CoA and higher including polyunsaturated-CoAs with the latter tissue, while ceramide synthase 4 (skin, liver, heart, adipose tissue and leukocytes) uses C18 to C22-CoAs. Ceramide synthases 5 (lung epithelia and brain gray and white matter) generates C16 (mainly) and C18 ceramides, and ceramide synthase 6 (intestine, kidney and lymph nodes) produces C14 and C16 ceramides. However, hydroxylation and the presence or otherwise of double bonds in the acyl-coAs do not appear to influence the specificity of the ceramide synthases. Also, the expression of mRNA expression for ceramide synthases does not always correlate with the fatty acid composition of sphingolipids in a particular tissue, suggesting that other factors are involved in determining which molecular species are formed. One such is acyl-coenzyme A-binding protein (ACBP), which facilitates the synthesis of ceramides containing very-long fatty acids and stimulates ceramide synthases 2 and 3 especially.
Insertion of the trans-double bond in position 4 to produce sphingosine occurs only after the sphinganine has been esterified in this way to form a ceramide as illustrated in Figure \(\PageIndex{11}\), with desaturation occurring at the cytosolic surface of the endoplasmic reticulum also. The desaturases were first characterized in plants, and this subsequently simplified the isolation of the appropriate enzymes in humans and other organisms. Two dihydroceramide desaturases have now been identified in animals and designated 'DEGS1 and DEGS2'. Both enzymes insert trans double bonds in position 4, but DEGS2 is a dual function enzyme that also acts as a hydroxylase to generate phytoceramides, i.e., to add a hydroxyl group on position 4. Distribution of the enzymes in tissues is very different, with DEGS1 expressed ubiquitously but highest in liver, Harderian gland, kidney and lung. DEGS2 expression is largely restricted to skin, intestine and kidney, where phytoceramides are more important. A considerable family of Δ4-sphingolipid desaturases has now been identified, and an early study by Stoffel and colleagues demonstrated that Δ4-desaturation involves first syn-removal of the C(4)- HR and then the C(5)-HS hydrogens. This appears to have been the first evidence that desaturases in general operate in this stepwise fashion.
The enzyme responsible for the insertion of the cis-14 double bond into sphinga-4-trans,14-cis-dienine is the fatty acid desaturase 3 (FADS3), which utilizes ceramides containing sphingosine as the precursor. The only other known activity of this enzyme is to insert a cis-double bond in position 13 of the CoA ester of vaccenic acid (11t-18:1) to produce the conjugated diene 11t,13c-18:2.
Synthesis of sphingoid bases de novo is essential in most organisms and inhibition of the biosynthetic pathways affects growth and viability. However, this can be tissue specific, as deletion of the liver-specific SPTLC2 in mice, was found to have no effect on liver function, while a comparable deletion of adipocyte-specific SPTLC1 caused major tissue defects. Presumably, the latter tissue is unable to take up enough sphingolipid from the circulation to remedy the problem. Deficiencies in SPTLC3 are related to dermal pathologies, and genetic variant of SPTLC3 are associated with dyslipidemia and atherosclerosis. The essentiality of sphingoid base synthesis in plants has been demonstrated in a similar manner in studies with mutants in which specific enzymes have been deleted.
Phytosphingosine and plant ceramides: Phytosphingosine is formed from sphinganine, produced as above, by hydroxylation in position 4, possibly via the free base in plants, although it can be formed both from sphinganine and a ceramide substrate in yeasts. A single sphinganine C4‑hydroxylase is present in yeast, but Arabidopsis has two such enzymes (SBH1 and 2), which are critical for growth and viability. Much remains to be learned of the processes involved, but it is known that the enzyme responsible is closely related to a Δ4 desaturase. Indeed, it has been shown that there are bifunctional Δ4‑desaturase/Δ4-hydroxylases in Candida albicans and mammals, especially in keratinocytes (DEGS2 discussed above) with which either 4‑hydroxylation or Δ4‑desaturation is initiated by removal of the proR C-4 hydrogen. Sphinganine linked to ceramide is the substrate for 4-hydroxylation in intestinal cells.
In Arabidopsis thaliana leaves, 90% of the sphingoid bases are phytosphingosine with a Δ8‑double bond. In plants in general, in addition to Δ4‑desaturation, two distinct types (20 gene products) of sphingoid Δ8-desaturase have been characterized that catalyse the introduction of a double bond at position 8,9 of phytosphingosine. These are evolutionarily distinct from the Δ4‑desaturases. One type produces the trans (E)-8 isomer mainly and the other mostly the cis (Z)-8 isomer, with overall the trans-isomer tending to predominate but dependent upon plant species. It appears that the trans isomer is formed when the hydrogen on carbon 8 is removed first, and the cis when carbon 9 is the point of attack. While the main group of Δ8-desaturases requires a 4‑hydroxysphinganine moiety as substrate, the second does not.
In Arabidopsis, three different isoforms of ceramide synthase have been identified and denoted LOH1, LOH2 and LOH3. Phytosphingosine is used efficiently by LOH1 and LOH3 (class II synthases), but only LOH2 (class I synthase) uses sphinganine efficiently; LOH2 and 3 prefer unsaturated long-chain bases. Marked fatty acid specificity is also observed with LOH2 showing almost completely specific for palmitoyl-CoA and dihydroxy bases, while LOH1 shows greatest activity for 24:0- and 26:0-CoAs and trihydroxy bases; none utilize unsaturated acyl-CoA esters efficiently. In plants, fatty acid desaturases and hydroxylases are also closely related, and sphingolipid fatty acid α-hydroxylation is believed to occur on the ceramide, as opposed to the free acyl chain. It is believed that the Δ8‑desaturase utilizes ceramide as the substrate and the channels the products selectively into the synthesis of complex sphingolipids, while Δ4‑desaturation channels ceramides for synthesis of glucosylceramide.
It has been established that long-chain bases with 4-hydroxyl groups are necessary for the viability of the filamentous fungus Aspergillus nidulans and for growth in plants such as A. thaliana. The presence of an 8E double bond confers aluminium tolerance to yeasts and plants, and it is important for chilling resistance in tomatoes. However, a trans-4 double bond in the sphingoid base does not appear to be essential for growth and development in Arabidopsis.
Fungal sphingoid bases: Fungi produce trans Δ8-isomers only, but Δ4- and Δ8-desaturases do not occur in the widely studied yeast S. cerevisiae. In the biosynthesis of sphingoid bases in fungi, the double bonds in positions 4 and 8 and the methyl group in position 9 are inserted sequentially into the sphinganine portion of a ceramide, the last by means of an S-adenosylmethionine-dependent methyltransferase similar to plant and bacterial cyclopropane fatty acid synthases. In S. cerevisiae the ceramide synthase is a heteromeric protein complex, containing three subunits, Lag1, Lac1, and Lip1, of which the first two are homologous proteins that feature eight transmembrane domains. In the yeast Pichia pastoris, there is a distinct ceramide synthase, which utilizes dihydroxy sphingoid bases and C16/C18 acyl-coenzyme A as substrates to produce ceramides. The long-chain-base components of the ceramide are then desaturated in situ by a Δ4‑desaturase and the fatty acid components are hydroxylated in position 2. Further desaturation of the long-chain base component by a Δ8-(trans)- desaturase occurs before the methyl group in position 9 is introduced by an S-adenosylmethionine-dependent sphingolipid C-9 methyltransferase. As a final step a trans-double bond may be introduced into position 3 of the fatty acid component. These ceramides are used exclusively for the production of glucosylceramides, and it is believed that a separate ceramide synthase encoded by a different gene produces the ceramide precursors for ceramide phosphorylinositol mannosides.
Viral sphingoid bases: The genome of an important marine virus (EhV) encodes for a novel serine palmitoyltransferase, which hijacks the metabolism of algal hosts to produce unusual hydroxylated C17 sphingoid bases; these accumulate in lytic cells of infected algae such as the important bloom-forming species Emiliania huxleyi. While this may seem a rather esoteric topic, viruses constitute a high proportion of the marine biome, and their control of the growth of algal blooms has global consequences.
Unesterified sphingosine: A cycle of reactions occurs in tissues by which sphingoid bases are incorporated via ceramide intermediates into sphingolipids, which are utilized for innumerable functions, before being broken down again to their component parts. It is worth noting that all the free sphingosine in tissues must arise by this route, in particular by the action of ceramidases on ceramides. Five such ceramidases are known with differing pH optima and varying subcellular locations. The levels of free sphingoids and their capacities to function as lipid mediators, as shown in Figure \(\PageIndex{12}\), are controlled mainly by enzymic re‑acylation to form ceramides, although some is acted upon by sphingosine kinases to produce sphingosine-1-phosphate.
Free sphingoid bases are absorbed by enterocytes following digestion of dietary sphingolipids in animals (including some from gut microorganisms), and while some of this is converted to complex sphingolipids, much is catabolized with the eventual formation of palmitic acid.
Catabolism of sphingosine and other long-chain bases occurs after conversion to sphingosine-1-phosphate and analogues. In yeasts, an alternative means of detoxification has been reported in which an excess of phytosphingosine is first acetylated and then converted to a vinyl ether prior to export from the cells.
Biological Functions of Unesterified Sphingoid Bases
The primary function of sphingoid bases is to serve as a basic component of ceramides and complex sphingolipids, where variations in their compositions can influence the physical and biological properties of these lipids. Independently of this in their free (unesterified) form, they are important mediators of many cellular events even though they are present at low levels only in tissues (typically 25 and 50 nM in plasma), with intracellular levels determined by hydrolysis by ceramidases or by the action of sphingosine kinases (sphingosine-1-phosphate production). In animal cells, they inhibit protein kinase C indirectly, possibly by a mechanism involving interference with the binding of activators of the enzyme, such as diacylglycerols or phorbol esters. In addition, sphingoid bases are known to be potent inhibitors of cell growth, although they stimulate cell proliferation and DNA synthesis. They are involved in the process of apoptosis in a manner distinct from that of ceramides by binding to specific proteins and regulating their phosphorylation. While sphingosine does not appear to participate in raft formation in membranes, it may rigidify pre-existing gel domains in mixed bilayers, although any such effects will be dependent on local concentrations and pH. It should be noted that some of the biological effects observed experimentally may be due to conversion to sphingosine-1-phosphate.
Free sphingosine has been implicated in various pathological conditions, and for example, plasma sphingosine levels are increased in hyperthyroidism and in patients with type 2 diabetes. Lysosomal storage of the lipid is an initiating factor in Niemann Pick type C disease, a neurodegenerative disorder, where it causes a change in calcium release leading to a buildup of cholesterol and sphingolipids. In the human adrenal cortex, sphingosine produced in situ by the acid ceramidase has a function in steroid production by serving as a ligand for steroidogenic factor 1 at the cell nucleus, which controls the transcription of genes involved in the conversion of cholesterol to steroid hormones. Unesterified sphingoid bases may have a protective role against cancer of the colon in humans. Thus, N,N‑dimethylsphingosine and dihydrosphingosine, like the deoxysphingoid bases, are known to induce cell death in a variety of different types of malignant cells. There is evidence that sphingadienes of plant and animal origin inhibit colorectal cancer in mouse models by reducing sphingosine-1-phosphate levels. In consequence, synthetic analogues of long-chain bases are being tested for their pharmaceutical properties.
Free sphingosine is believed to have a signaling role in plants by controlling pH gradients across membranes. In addition, free long chain bases (and the balance with the 1-phosphate derivatives) are essential for the regulation of apoptosis in plants.
Ceramides
Structure and Occurrence
The structure of ceramide is shown again in Figure \(\PageIndex{13}\).
Figure \(\PageIndex{13}\): Structure of ceramides (with varying fatty acids in ester link)
Ceramides consist of a long-chain or sphingoid base linked to a fatty acid via an amide bond. They are essential intermediates in the biosynthesis and metabolism of all sphingolipids including the complex sphingolipids in which the terminal primary hydroxyl group is linked to carbohydrate, phosphate, and so forth (sphingomyelin, glycosphingolipids and gangliosides) as shown in Figure \(\PageIndex{14}\).
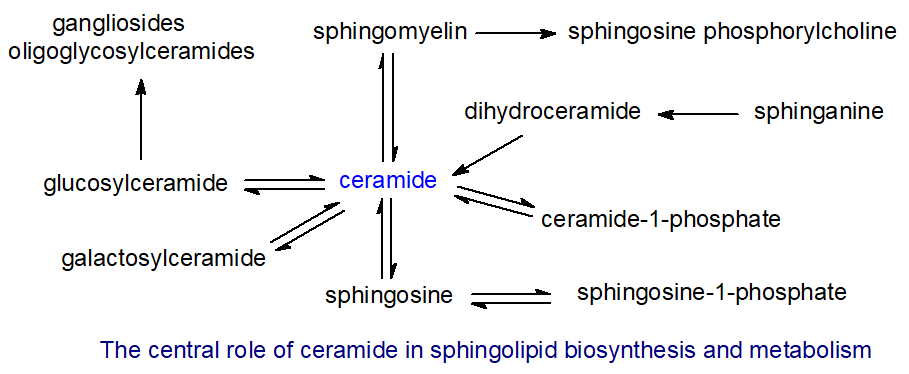
They are also the primary source of unesterified sphingoid bases and of the important biological mediators sphingosine-1-phosphate and ceramide-1-phosphate. At the last count, 33 different enzymes were known to participate in ceramide metabolism. While ceramides are rarely found as such at greater than trace amounts in tissues other than skin, they can exert important biological effects of their own at these low levels. They are present in membranes where they participate in the formation of raft domains.
Each organism and indeed each tissue may synthesize ceramides in which there are a variety of di- and trihydroxy long-chain bases linked to fatty acids. As discussed previously, the fatty acids consisting mainly of longer-chain (up to C24 or greater) saturated and monoenoic (mainly (n-9)) components, sometimes with a hydroxyl group in position 2. Other than in certain testicular cells, polyunsaturated fatty acids do not occur. More than 200 structurally distinct molecular species of ceramides have been characterized from mammalian cells. In plants, 2-hydroxy acids predominate sometimes accompanied by small amounts of 2,3-dihydroxy acids. Although small amounts of free ceramides are produced in all tissues as required for the specific biological functions described below, most is converted rapidly to more complex sphingolipids, including sphingomyelin (in animals) and the various glycosylceramides. The ceramides in skin are a remarkable exception to this rule, and as such they are discussed separately below.
A shorthand nomenclature simply combines those used conventionally for fatty acids and long-chain bases to denote molecular species of ceramides, including those as components of more complex lipids, e.g. N-palmitoyl-sphingosine is d18:1-16:0. Ceramides containing sphinganine are sometimes termed ‘dihydroceramides’.
Ceramide Biosynthesis
Ceramide production is complex and involves at least three pathways. Biosynthesis de novo takes place in the endoplasmic reticulum with palmitoyl-CoA and serine as the precursors for the long-chain base component, which is subsequently converted to ceramide. Biosynthesis of the very specific fatty acids in ceramides involving various chain elongases (ELOVL) requires consideration also. Alternative routes for ceramide production involve regeneration from complex sphingolipids. For example, in animals in the sphingomyelinase pathway, conversion of sphingomyelin into ceramides (and vice versa) occurs in the plasma membrane, Golgi and mitochondria. Finally, the polar moieties of complex glycosphingolipids can be removed by various hydrolytic enzymes in the lysosomal compartment to recover the ceramides (or their component parts) in a re-cycling/catabolic process. As these biosynthetic or metabolic pathways are located in different organelles, specific pools of ceramide and sphingolipids result with differing biological properties and functions.
Ceramide synthesis de novo: The first of these pathways is described in mechanistic. In brief in animals, sphinganine is coupled to a long-chain fatty acid to form dihydroceramide by means of one of six ceramide synthases in the endoplasmic reticulum mainly, before the double bond is introduced into position 4 of the sphingoid base. Of these, ceramide synthase 2 is most abundant and is specific for CoA esters of very-long-chain fatty acids (C20 to C26); it is most active in the central nervous system. Ceramide synthase 1 is specific for 18:0 and is located exclusively in brain and skeletal muscle, ceramide synthases 5 and 6 generate 16:0-containing ceramides, and ceramide synthase 3 is responsible for the unusual ceramides of skin and testes.
Figure \(\PageIndex{15}\) shows again the synthesis of ceramide from sphinganine and palmitoyl-CoA (a repeat of Figure \(\PageIndex{11}\)
Each synthase has six membrane-spanning domains and contains a characteristic motif with the specific structures required for catalysis and substrate binding that are essential for its activity, and they have been shown to differ primarily in an 11-residue sequence in a loop between the last two putative transmembrane domains. In addition to separate transcriptional regulation of each of these enzymes, ceramide synthase activity is modulated by many different factors including reversible dimerization, while ceramide synthase 2 has a sphingosine-1-phosphate binding motif and this lipid may inhibits its activity. Acyl-coenzyme A-binding protein (ACBP) facilitates the synthesis of ceramides containing very-long fatty acids and stimulates ceramide synthases 2 and 3 especially.
Most of the ceramides generated in this way are rapidly utilized for synthesis of complex sphingolipids, especially sphingomyelin and hexosylceramides, to ensure that cellular ceramide concentrations are regulated to control their biological activities. In mammalian cells, most complex glycerolipids are synthesized in the endoplasmic reticulum prior to their transport to their final subcellular locations, but the process is rather different for sphingolipids. Ceramide is synthesized on the cytoplasmic leaflet of the endoplasmic reticulum, but subsequent formation of complex sphingolipids occurs in the Golgi apparatus, and a key cytoplasmic protein, ceramide transporter or 'CERT' (CERamide Trafficking), mediates the transport of ceramide between these organelles in a non-vesicular manner. It has a number of distinct functional domains, including an N-terminal phosphatidylinositol-4-monophosphate (PI(4)P)-binding or Pleckstrin homology (PH) domain, which targets the Golgi apparatus, and a C-terminal ‘START’ domain, which can recognize ceramide species with the natural D-erythro stereochemistry, including dihydroceramide and phytoceramide (but not sphingosine), and holds them within in a long amphiphilic cavity by hydrogen bonding with all three polar atoms of the sphingoid motif. There is also a short peptide motif (FFAT) that recognizes a specific protein in the endoplasmic reticulum. There is sufficient flexibility in the body of the protein to enable transfer of ceramide from the endoplasmic reticulum to the Golgi without free movement through the cytosol.
Very-long-chain ceramides containing 24:0 or 24:1 fatty acids turn over much more rapidly in animal cells than those containing 16:0 or 18:0 fatty acids, because of the more rapid conversion of the former into complex sphingolipids, where they may regulate the levels and perhaps the biological functions of the latter. In contrast, ceramides containing d16:1 and d18:1 sphingoid bases turnover at similar rates so do not affect the flux of ceramides through these pathways. The CERT protein is a major factor in this specificity, as it extracts ceramides from membrane bilayers with a preference for those required for synthesis of complex sphingolipids. Removal of ceramide by this process provides the gradient that enables the process to continue, and prevents an accumulation of ceramide in the endoplasmic reticulum that might otherwise be disruptive to the membrane and even cause cell death. While the transfer process itself is not dependent on ATP, the overall process requires ATP, possibly to keep PI(4)P in a phosphorylated form, and the multiple factors that control the biosynthesis of this lipid must also influence sphingolipid metabolism.
As a neutral lipid, ceramide can flip readily across membrane leaflets, and this is also necessary for the synthesis of sphingomyelin, which occurs on the lumen of the Golgi. The pool of ceramide utilized for the synthesis of glycosylceramide is delivered to the Golgi by a separate transport mechanism that also does not require ATP. In addition, some ceramide synthesis occurs in mitochondria although this has the potential to lead to cell death. Regulation of ceramide and subsequent sphingolipid biosynthesis is crucial as an excess of sphingolipids can be toxic, while reduced synthesis can inhibit cell proliferation.
Some ceramides are transported from the liver to other tissues in plasma lipoproteins, but especially subclasses HDL2 and HDL3, i.e. those containing apolipoprotein B. There is a suggestion that transport of ceramides via lipoproteins could be a paracrine mechanism to regulate the metabolism of other cells.
Ceramides are also produced during the catabolism of other complex sphingolipids, and especially by the action of one or other of the sphingomyelinases or of phospholipase C on sphingomyelin in animal tissues as part of the 'sphingomyelin cycle' as shown in Figure \(\PageIndex{16}\).
Many agonists including chemotherapeutic agents, tumor necrosis factor-alpha, 1,25-dihydroxy-vitamin D3, endotoxin, gamma-interferon, interleukins, nerve growth factor, ionizing radiation and heat stimulate hydrolysis of sphingomyelin to produce ceramide. In addition, reversal of the sphingomyelin synthesis reaction may generate ceramide, and some may be produced by operation of the enzyme ceramidase in reverse (see next section). Such reactions are much more rapid than synthesis de novo, so they are of special relevance in relation to the signaling functions of ceramides, especially when they occur at the plasma membrane. For example, in this context, the acid sphingomyelinase may be especially important by generating the ceramides that initiate the train of events that leads to apoptosis (see below).
Glycosphingolipids can be hydrolyzed by glycosidases to ceramides also in tissues, but the process tends to be less important in quantitative terms (other than in skin). The key enzymes of sphingolipid metabolism were first characterized from the yeast Saccharomyces cerevisiae, and these were found to be sufficiently similar to the corresponding enzymes in mammals to facilitate their study in the latter.
As discussed above, there are specific ceramide synthases that utilize specific fatty acids for ceramide biosynthesis in animals, and knowledge is slowly being acquired of how these are compartmentalized and regulated within cells. Thus, the synthesis and subsequent catabolism of ceramides involves a complex web of at least 28 distinct enzymes, including six ceramide synthases and five sphingomyelinases, which are all products of different genes. Each of these enzymes may produce distinctive molecular species of ceramides with their own characteristic biological properties. It has been determined that ceramide species containing very-long-chain fatty acids (C24) turnover more rapidly than those containing C16/18 components.
Ceramide Catabolism
In animals, ceramide metabolism and function are controlled in part by the action of ceramidases, which cause hydrolysis forming sphingoid bases and free fatty acids, and indeed this is the only route to the formation of unesterified sphingosine. This is illustrated in Figure \(\PageIndex{17}\).
Five such enzymes are known in humans, classified according to their pH optima, i.e. acid (‘ASAH1’), neutral (‘ASAH2’, which differs between humans and animals), and alkaline (three enzymes - ‘ACER1 to ACER3’), with differing cellular locations and fatty acid specificities and with the potential to affect distinct signaling and metabolic events. The acid ceramidase is of particular importance, and aberrations in its synthesis or activity is involved in several human disease states, including the rare autosomal-recessive Farber disease where there is a deficiency in the enzyme so ceramide accumulates; ceramide containing 26:0 in the blood is considered to be a biomarker for diagnosis of the disease. ASAH1 is located in the lysosomes and hydrolyses ceramides with small to medium-chain fatty acid components (C6 to C18) most efficiently. The neutral ceramidase is located in the plasma membrane and Golgi, especially of intestinal epithelial cells and colorectal tissues, and prefers long-chain components (C16 to C18); it also catalyzes the reverse reaction, and this may be a means of ceramide synthesis in mitochondria. ACER1 and ACER2 are found in the endoplasmic reticulum and Golgi, respectively, and they prefer species with very-long-chain acyl groups. ACER3 is present in both the endoplasmic reticulum and Golgi; it has a marked specificity for ceramides, dihydroceramides, and phytoceramides linked to unsaturated long-chain fatty acids (18:1, 20:1 or 20:4) in vitro at least. Neutral/alkaline ceramidase activity has also been found in mitochondria and nuclei.
In Arabidopsis, an alkaline ceramidase (AtACER) can hydrolyze phytosphingosine-containing ceramides, and a related enzyme from rice has a preference for d18:1Δ4-ceramide; the latter can function in reverse to increase the content of C26- and C28-phytoceramides. Several neutral ceramidases (AtNCERs) have been identified, but there does not appear to be an equivalent to the acid ceramidase in plants. Ceramidases are also present in lower organisms such as Pseudomonas aeruginosa and slime molds, where they are secreted proteins rather than integral membrane enzymes. A neutral ceramidase only is found in prokaryotes, including some pathogenic bacteria.
Sphingoid bases released by the action of acid ceramidase can escape from the lysosomes and be re-utilized for ceramide biosynthesis through the action of a ceramide synthase. This has been termed the ‘salvage’ pathway and is important in both quantitative and biological terms. For example, it has been estimated that it contributes from 50 to 90% of sphingolipid biosynthesis. The biological functions of ceramides are discussed below, but there are reasons to believe that ceramides derived from the salvage pathway are spacially and thence functionally distinct from those synthesized de novo. In addition, sphingoid bases released in this way have their own biological functions, which includes utilization for the synthesis of the biologically important metabolite sphingosine-1-phosphate. Therefore, regulation of ceramidase action is central to innumerable biological processes in animals.
Biological Functions of Ceramides
The role of ceramides in the biosynthesis of complex glyco- and phospho-sphingolipids are discussed elsewhere in this text. Ceramides, like other lipid second messengers in signal transduction, are produced rapidly and transiently in response to specific stimuli in order to target specific proteins, for example to activate certain serine/threonine protein kinases or phosphatases. They may also regulate cellular processes by influencing membrane properties. While they can be produced by synthesis de novo for such functions, activation of one of the sphingomyelinases under physiological stress or other agents is a more rapid means of generation in animal tissues at least. In fact, ceramides appear to be formed under all conditions of cellular stress by a multiplicity of activators in eukaryotic organisms. However, it should be noted that ceramides with different fatty acid and long-chain base (molecular species) compositions are formed in different compartments or membranes of the cell by various mechanisms over different time scales and potentially with distinct functions. The biological functions of those ceramides containing medium-chain (up to C14), long-chain (C16 and C18), and very-long-chain (C20 and longer) fatty acids, in particular, may have to be considered separately.
Physical properties: Unsaturation in the sphingoid backbone augments intramolecular hydrogen bonding in the polar region, which permits a close packing of ceramide molecules and a tight intramolecular interaction in membranes. A further important factor in this context is the length of the fatty acyl moiety, as shorter-chain ceramides tend to produce a positive curvature in a lipid monolayer, while long-chain molecules have the opposite effect and possess a marked intrinsic negative curvature that facilitates the formation of inverted hexagonal phases as well as increasing the order of the acyl chains in bilayers. By their interactions with ion channels, ceramides influence the permeability of membranes and render bilayers and cell membranes permeable to solutes that vary from small- up to protein-size molecules.
While ceramides are minor components of membranes in general, their physical properties ensure that they are concentrated preferentially into lateral liquid-ordered microdomains (a distinct form of 'raft' termed ‘ceramide-rich platforms’), although these effects are again chain-length specific. These domains differ appreciably in composition from those rafts enriched in sphingomyelin and cholesterol, and ceramides containing C12 to C18 fatty acids can in fact displace cholesterol from rafts to modify their physical properties. Ceramides are generated within rafts by the action of acid sphingomyelinase, causing small rafts to merge into larger units and modifying the membrane structure in a manner that is believed to permit oligomerization of specific proteins such as cytokines and death receptors. Ceramides are also essential for the formation and/or secretion of exosomes by facilitating or inducing membrane curvature. In contrast, sphingosine, sphingosine-1-phosphate and ceramide-1-phosphate do not facilitate raft formation.
Through the medium of these modified rafts, ceramides are able to function in signal transduction. Specific receptor molecules and signaling proteins are recruited and cluster within such domains, thereby excluding potential inhibitory signals, while initiating and greatly amplifying primary signals. It is believed that ceramide-rich platforms amplify both receptor- and stress-mediated signaling events and thence may influence various disease states. Ceramide-enriched membrane domains formed in response to sphingomyelinase activity are sites for endocytic uptake of pathogens because of a concentration of pathogen receptors and signaling complexes, and in particular these can enhance viral infections, including Norovirus, Japanese encephalitis virus, Ebola and possibly SARS-CoV-2. However, elevated levels of ceramide inhibit cellular uptake of the HIV virus.
Although ceramides and diacylglycerols have structural similarities, their occurrence, location, and behavior in membranes are different. Ceramides cross synthetic lipid bilayers relatively quickly in vitro, but it is not clear whether they can flip across more complex biological membranes equally readily, especially in the ceramide-rich platforms. Restricted flipping could have important effects on the signaling role of ceramides in that those generated by different enzymes on each side of a membrane could have distinct functions.
Enzyme activation: In general, ceramides tend to modify intracellular signaling pathways to slow anabolism and promote catabolism. Amongst a wide range of biological functions in relation to cellular signaling, ceramides are especially important in triggering apoptosis, and they have also been implicated in the activation of various protein kinase cascades, dependent on the site of generation. The mechanism of these interactions is the subject of intensive study at present, but in relation to the latter, two intracellular targets for ceramide action of special importance have been discovered – at least two protein phosphatases (ceramide-activated protein phosphatases) and a family of protein kinases (ceramide-activated protein kinases). For example, the phosphatase may be involved in the regulation of glycogen synthesis, insulin resistance, and response to apoptotic stimuli. Ceramides generated by the action of sphingomyelinase and by synthesis de novo are both important to the process, while ceramidases have contrasting effects in these and other biological effects of ceramides.
Apoptosis: The role of ceramides in the regulation of apoptosis, and cell differentiation, transformation, and proliferation has received special attention. Apoptosis is a normal process, which occurs in response to oxidative stress in particular, in which a cell can be considered to actively ‘commit suicide’. It is essential for many aspects of normal development and is required for maintaining tissue homeostasis. There are two pathways - 'extrinsic' initiated in the plasma membrane by ligation of so-called 'death factors', such as the tumor necrosis factor-α (TNF-α), and 'intrinsic' induced by external actions in mitochondria, e.g. by DNA damage, oxidation or radiation injury. Although the mechanism of the ceramide interaction with these pathways is uncertain, it is clear that a cascade of reactions is initiated that culminates in the release of intracellular proteases of the caspase family to promote apoptosis. In dysfunctional mitochondria, one mechanism involves the formation of channels in the membrane that enable the release of specific mitochondrial proteins that include caspases. Ceramides with fatty acids of differing chain lengths are believed to function in different ways, and 16:0-ceramide generated by ceramide synthase 6 is especially pro-apoptotic, for example, while ceramides with very-long-chain fatty acids accumulate in necroptosis, a form of apoptosis. On the other hand, ceramides containing 2-hydroxy acids in keratinocytes appear to be protective against apoptosis. Ceramides induce the related process of cellular senescence also.
Failure to properly regulate apoptosis can have catastrophic consequences, and many disease states, including cancer, diabetes, neuropathies, Alzheimer's disease, Parkinson's disease, and atherosclerosis, are thought to arise from the deregulation of apoptosis. For example, ceramides have been implicated in the actions of TNF-α and in the cytotoxic responses to amyloid Aβ peptide, which are involved in Alzheimer’s disease and neurodegeneration. In addition, ceramides appear to be involved in many aspects of the biology of aging and of male and female fertility. These effects may hold implications for diseases associated with obesity and insulin resistance, including again diabetes and cardiovascular disease.
Similarly, ceramides are intimately involved in the induction of autophagy, the 'maintenance' process by which cellular proteins and excess or damaged organelles are removed from cells by engulfing them in a membrane-enclosed cellular compartment called the phagosome. In particular, maturing phagosomes are enriched in very-long-chain ceramides. While this process is beneficial in that it aids the recycling of cellular nutrients, the presence of excess ceramide can lead to unnecessary apoptosis.
As animals and plants have multiple isoforms of ceramide synthase that are specific for the chain length of the base and fatty acid, it has been suggested that ceramides containing different fatty acids have distinct roles in cellular physiology. In particular, C16 ceramide appears to be especially important in apoptosis in non-neuronal tissues, while C18 ceramide has growth-arresting properties and may be involved in apoptosis in some carcinomas treated with chemotherapy agents. In addition, a transferase has been identified that transfers the acetyl group from platelet-activating factor to sphingosine with a high specificity. The product, N-acetylsphingosine - the simplest of all ceramide molecules, has signaling functions that are distinct from those of the parent lipids or of other ceramides; it does not enter the salvage pathway in cancer cells in vitro and is cytotoxic.
In contrast, the ceramide metabolite, sphingosine-1-phosphate, has opposing effects on cell survival and proliferation. As ceramide and sphingosine-1-phosphate are inter-convertible via sphingosine as an intermediate, which also has pro-apoptopic activity, the balance between these lipids and with ceramide-1-phosphate is obviously of great metabolic importance. It has been termed the ‘sphingolipid-rheostat’, as illustrated in Figure \(\PageIndex{18}\).
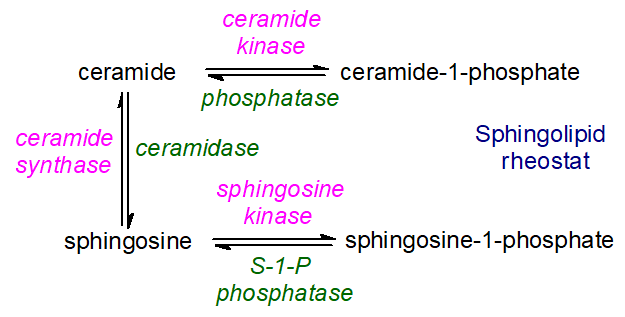
Plants: Comparatively little information is available on the role of ceramides in cell signaling in plants, but there are suggestions that sphingolipid catabolic products may be linked to programmed cell death, signal transduction, membrane stability, host-pathogen interactions, and stress responses. For example, there is evidence that enhanced synthesis of ceramides with very-long-chain fatty acids and trihydroxy sphingoid bases by ceramide synthases LOH1 and LOH3 promotes cell division and growth, while in contrast, accumulation of the ceramide species C16 fatty acid with a dihydroxy sphingoid base, due to LOH2 overexpression, leads to plant dwarfing and programmed cell death. Ceramides aggregate in rafts in plant membranes, together with other sphingolipids and sterols, as in animal tissues. Similarly, in the yeast S. cerevisiae, widely used as a model organism, it has been reported that ceramide species with different N-acyl chains and sphingoid bases are involved in the regulation of different sets of functionally related genes.
Skin Ceramides
The mammalian skin forms the protective barrier between the internal tissues of the host and the hostile external environment, which can include chemicals, ultraviolet light, mechanical damage, and pathogenic microorganisms, while preventing the loss of water and electrolytes. It consists of stratified layers of increasingly differentiated cells or keratinocytes of which the basal layer is responsible for the renewal of the tissue but begins to migrate upwards and differentiate, while accumulating specific lipids and proteins that change the cellular architecture. Eventually, the keratinocytes lose their nucleus and become flattened structures of insoluble protein surrounded by lipids termed ‘corneocytes’ in the outermost impermeable layer or stratum corneum. By secreting peptides and proteins that possess antimicrobial activity, keratinocytes add to the defensive capability of skin against commensal microorganisms and opportunistic pathogens, and this is reinforced by lipid mediators such as free sphingoid bases and eicosanoids in the stratum corneum and free fatty acids in sebum.
The stratum corneum contains high levels of ceramides (as much as 50% of the total lipids), including O-acylceramides, which exist both in the free form and linked by ester bonds to structural proteins. They are present mainly in the extracellular domains (interstices) and are accompanied by nearly equimolar amounts of cholesterol and free fatty acids, a ratio that is believed to be essential for the normal organization of the tissue into the membrane structures that are responsible for the functioning of the epidermal barrier. In contrast to other biological membranes, the lipid organization in the membranes of skin consists of two lamellar phases, which form crystalline lateral phases mainly, with repeat distances of approximately 6 and 13 nm. Small sub-domains of lipids in a liquid phase may also exist.
Some of these skin ceramides have distinctive structures not seen in other tissues, and many different forms are commonly recognized. They can contain the normal range of longer-chain fatty acids (a), e.g. formula 1 in the figure, some with hydroxyl groups in position 2 (a*), e.g. formula 2, linked both to dihydroxy bases with trans-double bonds in position 4 or to trihydroxy bases. This is illustrated in Figure \(\PageIndex{18}\).
In addition, there are O‑acyl ceramides in which a unique very-long-chain fatty acid component (typically C30 or C32) has a terminal hydroxyl group, and this may be in the free form or esterified with linoleate (c), e.g., formulae 3 and 4; the sphingoid base can be either di- (b) or trihydroxy (b*), e.g., formula 4; the latter is not a common feature in sphingolipids of animal origin, and can include both phytosphingosine and the unique 6‑hydroxy-4-sphingenine in human epidermis. Ceramides of type 1 in which the 1-O-hydroxyl group of the sphingoid base is acylated by a very-long-chain fatty acid are also present (1‑O‑acylceramides - illustrated above); these comprise 5% of the total ceramides in the epidermis of mice and humans and comprise as much as 700 molecular species. In all, 15 classes of free ceramides and 3 classes of covalently bound ceramides with up to 1700 distinct molecular species have been identified. Such lipids were first studied in detail in the skin of the pig as a convenient experimental model, but they have been characterized in humans and rats. In addition, several molecular forms of glucosylceramide, based on similar ceramide structures, have been characterized in skin, and these are also essential for its proper function.
Depending on the particular layer of the skin (keratinocytes, stratum corneum, etc.), the lipid composition can vary. These lipids have an obvious role in the barrier properties of the skin, limiting the loss of water and solutes and at the same time preventing the ingress of harmful substances. As the aliphatic chains in the ceramides and the fatty acids are mainly non-branched long-chain saturated compounds with a high melting point and a small polar head group, the lipid chains are mostly in a solid crystalline or gel state, which exhibits low lateral diffusional properties and low permeability at physiological temperatures. There is a report that the stratum corneum layer of the skin has a water permeability only one-thousandth that of other biomembranes, for example. Natural and synthetic ceramides are now commonly added to cosmetics and other skin care preparations.
Most steps in the biosynthesis of ceramides linked to ω-O-acylated fatty acids occur in the endoplasmic reticulum of keratinocytes. First, fatty acid synthesis of very-long-chain (and ultra-long-chain, ≥C26) acyl-CoA de novo must take place, requiring the chain-elongation enzymes ELOVL1 and ELOVL4. Desaturation can occur, and importantly oxidation in the 2 (α) and terminal (ω) positions. The ω‑hydroxylation step requires an enzyme of the cytochrome P450 family, designated CYP4F22, of the kind involved in the synthesis of hydroxy-eicosatetraenoic acids (HETE). Mutations are a cause of lamellar ichthyosis, and knockout mice deficient in the equivalent enzyme were found to die within 8 hours of birth.
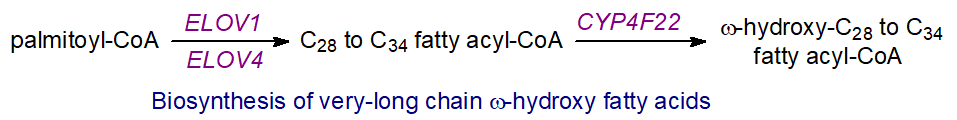
Ceramides are first synthesized by ceramide synthase 3 (CERS3), which has a high specificity for very-long-chain fatty acids (>C26) with the incorporation of the ω‑hydroxy fatty acid. This is acylated with linoleate by the action of an unusual enzyme related to the phospholipase A family, PNPLA1, which catalyzes esterification by first releasing linoleate from triacylglycerols in the skin while acting as an acyltransferase to link the linoleate directly to the ω-hydroxyl moiety of the ultra-long chain fatty acid. PNPLA1 is unique among phospholipases in that it is involved in the metabolism of sphingolipids rather than glycerophospholipids and catalyzes transacylation rather than hydrolysis. In addition, some linoleate for this purpose is released from triacylglycerols by the action of the adipose tissue lipase aided by a protein ABHD5. This process is vital for proper skin barrier function and keratinocyte differentiation, as mice with defective triacylglycerol biosynthesis and metabolism, including a deficiency of the acyl-CoA synthase ACSL1, are unable to synthesis ω‑O‑acylceramides and have an impaired skin barrier. Mutations in the human PNPLA1 gene are believed to be the cause of autosomal recessive disease congenital ichthyosis.
The resulting ceramides are converted to the complex sphingolipids sphingomyelin and especially glucosylceramide, which are transferred with the aid of ATP-binding cassette (ABC) transporters together with degradative enzymes into the stratum corneum via specific organelles termed 'lamellar bodies.' These organelles must fuse with the apical plasma membrane of the outermost cell layer of the epidermis in order that their contents can be secreted. It is only then that the final step of hydrolysis of the lipid precursors occurs in the extracellular spaces of the stratum corneum, i.e. ceramides are generated from sphingomyelin by the action of acid sphingomyelinase and from glucosylceramides by β-glucocerebrosidase. This mechanism ensures that ceramides, with their potentially harmful biological activities, never accumulate within nucleated cells.
Eventually, ceramides with a terminal ω-hydroxyl group in the fatty acyl moiety are bound covalently to the proteins of the cornified envelope, especially to involucrin. This is illustrated in Figure \(\PageIndex{19}\).
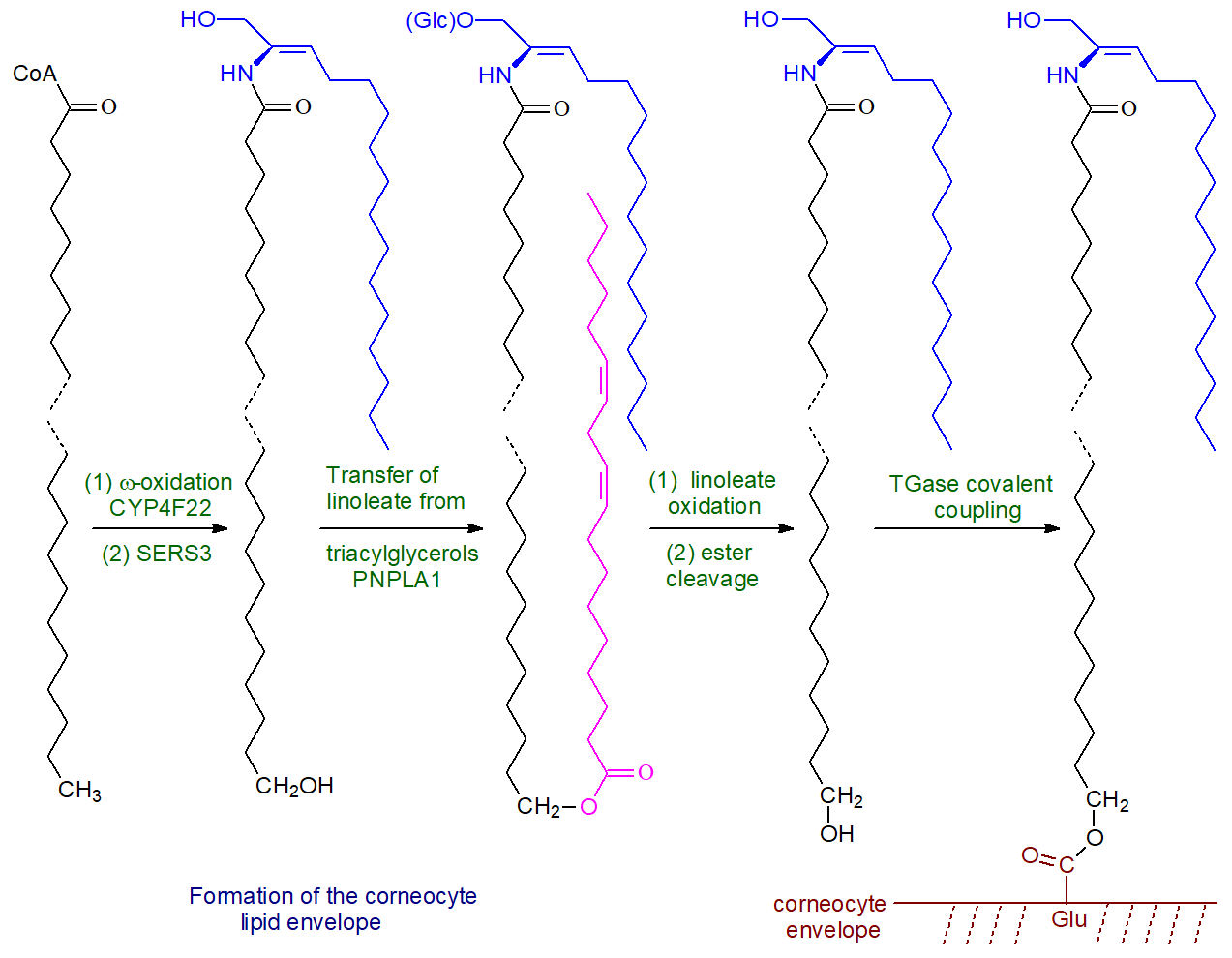
Sphingomyelin and Related Sphingophospholipids
Structure and Occurrence of Sphingomyelin
Sphingomyelin or ceramide 1-phosphocholine consists of a ceramide unit with a phosphorylcholine moiety attached to position 1 of the sphingoid base component. It is thus the sphingolipid analog of phosphatidylcholine, and like that lipid it is zwitterionic. The d18:1/16:0 molecular species is illustrated as an example in Figure \(\PageIndex{20}\).
Sphingomyelin is primarily of animal origin and is a ubiquitous component of all animal cell membranes, from mammals to nematodes (and in a few protozoa), where it is by far the most abundant sphingolipid. Indeed, it can comprise as much as 50% or more of the lipids in certain tissues, though it is usually lower in concentration than phosphatidylcholine. For example, it makes up about 10% of the lipids of the brain, where it is a key constituent of myelin, but 70% of the phospholipids of the human lens. Like phosphatidylcholine, sphingomyelin tends to be in greatest concentration in the plasma membrane of cells (up to 20%), and in the endocytic recycling compartment and trans Golgi network. It is also abundant in the nucleus where it is the main phospholipid associated with chromatin, but there is very little in the endoplasmic reticulum (2 to 4%) and even less in mitochondria. All the sphingomyelin in human erythrocyte membranes is in the outer leaflet, and ~90% of that in the plasma membrane of nucleated cells is in the outer leaflet. All lipoprotein fractions in plasma contain appreciable amounts of sphingomyelin with a higher proportion in the VLDL/LDL. Sphingomyelin is the single most abundant lipid in erythrocytes of most ruminant animals, where it replaces phosphatidylcholine entirely. In this instance, there is known to be a highly active phospholipase A that breaks down the glycerophospholipids, but not sphingomyelin.
Sphingomyelin is not synthesized in plants or fungi, which produce the sphingophospholipid ceramide phosphoinositol and related lipids instead, or in bacteria, and its evolutionary significance is a matter for speculation. However, a number of bacteria and viruses utilize sphingomyelin or its metabolism in their hosts for growth and viability.
Sphingosine is usually the most abundant long-chain base constituent, together with sphinganine and C20 homologues, although other bases can be present, especially in ruminant animals. In contrast, sphinganine is the major sphingoid base in the sphingomyelin of human lens membranes, linked mainly to 16:0. Typically, the fatty acids are very-long-chain saturated and monounsaturated, including odd-numbered components. In comparison to the glycosphingolipids, 2‑hydroxy acids are only rarely detected and then in small amounts, but they are found in testes, spermatozoa, kidney and skin sphingomyelin, for example. The absolute proportions of each fatty acid and sphingoid base can vary markedly between tissues and species, and some of the variability in compositions can be seen from the data in Table \(\PageIndex{3}\) and Table \(\PageIndex{4}\) below.
| Table \(\PageIndex{3}\): Fatty acid compositions of sphingomyelin (wt % of the total) in some animal tissues. | ||||||||||
| Source | Fatty acids | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 20:0 | 22:0 | 22:1 | 23:0 | 23:1 | 24:0 | 24:1 | |
| Egg | 66 | 10 | 1 | 4 | 6 | 1 | 2 | - | 5 | 3 |
| Bovine brain | 3 | 42 | - | 6 | 7 | 3 | 3 | 3 | 6 | 27 |
| Cow's milk | 14 | 3 | 1 | 1 | 22 | - | 32 | - | 19 | 5 |
| Adapted from Ramstedt, B. et al. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem., 266, 997-1002 (1999); DOI. | ||||||||||
| Table \(\PageIndex{4}\): Long-chain base compositions of sphingomyelin (wt % of the total) in some animal tissues. | |||||||
| Source | Sphingoid base | ||||||
|---|---|---|---|---|---|---|---|
| d16:0* | d17:0 | d17:1 | d17:1-methyl | d18:0 | d18:1 | d19:0 | |
| Egg | 7 | 93 | |||||
| Bovine brain | 19 | 81 | |||||
| Cow's milk | 9 | 15 | 8 | 11 | 10 | 44 | 3 |
| Also from Ramstedt, B. et al. Eur. J. Biochem., 266, 997-1002 (1999); DOI. * d = dihydroxy base |
|||||||
Palmitic acid (16:0) is the most common fatty acid component of sphingomyelin in peripheral cells of mammals, while stearic acid (18:0) is more abundant in neural tissue, but this only hints at the potential complexity as there can be variability within tissues. For example, about 60% of the fatty acids of the sphingomyelin of the grey matter of the human brain consist of stearic acid (18:0), while lignoceric (24:0) and nervonic (24:1) acids make up 60% of the corresponding lipid of white matter, although this is dependent on the stage of development. During the first two years of life, the 18:0 concentration in sphingomyelin of white matter decreases from 82% to 33%, while the proportions of 24:0 and 24:1 increase. This pronounced shift from long-chain to very-long-chain sphingomyelins is not observed in the cerebral cortex. Approximately 100 molecular species of sphingomyelin have been detected in human plasma. Although polyunsaturated fatty acids such as arachidonic acid are rarely present, they have sometimes been mistakenly identified in the literature. Exceptions are the sphingomyelins of testes and spermatozoa, which contain very-long-chain polyunsaturated fatty acids (up to 34 carbon atoms), the major components being 28:4(n-6) and 30:5(n-6) with a proportion having hydroxyl groups in position 2.
Biosynthesis, Metabolism and Function of Sphingomyelin
The biosynthesis of sphingomyelin is distinct from that of phosphatidylcholine and indeed depends upon it, as it involves the transfer of phosphorylcholine from phosphatidylcholine to ceramide, synthesized in the endoplasmic reticulum, with the liberation of 1,2-diacyl-sn-glycerols. as illustrated in Figure \(\PageIndex{21}\).
The reaction is catalyzed by a ceramide choline-phosphotransferase (sphingomyelin synthase or SMS) and takes place primarily on the luminal side of the trans-Golgi but also in the plasma membrane, with two related enzymes each with six transmembrane domains and their N- and C-termini facing the cytosol, i.e., SMS1 and SMS2. Both enzymes are present in the Golgi, but only SMS2 is in the plasma membrane (facing the extra-cellular space in this instance) and may be necessary for the formation of raft domains (see below). SMS2 is also present in the membranes of nuclei from rat liver cells. It is noteworthy that in the absence of ceramide, both SMS1 and 2 have phospholipase C activity, and so may regulate the steady-state levels of phosphatidylcholine and diacylglycerols as well as that of sphingomyelin. The reaction does not use free phosphorylcholine or CDP-choline as a donor.
Figure \(\PageIndex{22}\) shows an interactive iCn3D model of the AlphaFold model of human Golgi membrane phosphatidylcholine:ceramide cholinephosphotransferase 1, also called sphingomyelin synthase 1 (Q86VZ5).
Figure \(\PageIndex{22}\): . Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...v6s5wHRgPXKJC6
The gray helices are the transmembrane helices. A cytoplasmic protein:protein interaction domain called SAM (sterile alpha motif) is shown in magenta. The other cytoplasmic C-terminal domain is shown in cyan Obviously much of the cytoplasmic domain is disordered in this computational structure. Side chains involved in binding phosphatidylcholine are shown as sticks colored CPK. Note that two, D95 and S97 are located in a disordered section in this model but would close in the actual active site in the actual structure.
It has been proposed that Asp101 (95 in the AlphaFold structure) deprotonates Arg220 (214 in the model), which then acts as a nucleophile which attacks the phosphate group of phosphatidylcholine. Given the very high pKa of arginine, this mechanism, if true, is somewhat unique. Phosphocholine is linked to ceramide to produce sphingomyelin. Three key and extremely conserved amino acids in the active site are Asp101, Arg220 and Asn358.
A specific ceramide transport molecule (CERT) is important to the reaction with SMS1 in that it transfers ceramide from the cytosolic surface of the endoplasmic reticulum to the trans-Golgi in an ATP-dependent and non-vesicular manner. Much of the sphingomyelin produced in the Golgi is then delivered to the apical plasma membrane by a vesicular transport mechanism. Sphingomyelin synthesis is regulated in part by phosphatidylinositide metabolism and is connected to sterol homeostasis through the oxysterol binding protein (OSBP).
SMS2 in the plasma membrane is not dependent on CERT-mediated ceramide delivery, but is believed to convert ceramide produced locally by a sphingomyelinase back to sphingomyelin; this may be an important protective mechanism for the cell. The location of the enzymes explains the enrichment of sphingomyelin in specific membranes and the sidedness, i.e., the luminal trans-Golgi and the outer leaflet of the plasma membrane, while ceramide reaching the cis-Golgi is utilized for the synthesis of glucosylceramide. As the nature of the molecular species of sphingomyelins produced differs appreciably from that of the ceramide precursors, the sphingomyelin synthases must have considerable substrate specificity. The reaction can be reversible, using sphingomyelin to generate ceramide for specific signaling functions. It is evident that sphingomyelin biosynthesis forms a link between the sphingolipid signaling pathway (pro-apoptotic - see below) and that of glycerolipids via the mitogenic diacylglycerol by‑products. Although the importance of this production relative to that via phosphatidylinositol is not known, it is possible that it is significant locally at the external leaflet of the plasma membrane.
An alternative pathway of sphingomyelin synthesis has been demonstrated in the endoplasmic reticulum in which ceramide is first converted to ceramide phosphoethanolamine via transfer of the head group from phosphatidylethanolamine, followed by stepwise methylation of the ethanolamine moiety. However, the physiological significance of this pathway has yet to be established.
It was long thought that the only function of sphingomyelin was to serve as a substitute for phosphatidylcholine as a building block of membranes, i.e., by forming a stable and chemically resistant outer leaflet of the plasma membrane lipid bilayer. For example, it may limit the ingress of oxygen and thence oxidation of adjacent unsaturated acyl chains. While this is certainly one of its functions, the apparent similarity between phosphatidylcholine and sphingomyelin is superficial, and there are great differences in the hydrogen bonding capacities and physical properties of the two lipids. For example, sphingomyelin has an amide bond at position 2 and a hydroxyl at position 3 of the sphingoid base, both of which can participate in hydrogen bonding, while the trans double bond also appears to assist intermolecular interactions in membranes. Indeed, the first five carbon atoms of the sphingoid base in sphingolipids constitute a key feature that has been termed the ‘sphingoid motif’, which facilitates a relatively large number of molecular interactions with other membrane lipids, via hydrogen-bonding, charge-pairing, hydrophobic and van der Waals forces. With phosphatidylcholine, in contrast, the two ester carbonyl groups can act only as hydrogen acceptors. The degree of unsaturation of the alkyl moieties in each lipid is very different, and this gives them dissimilar packing properties in membranes.
It is now recognized that sphingomyelin and other sphingolipids have a strong tendency to interact with proteins and cholesterol, often via strong van der Waals interactions and hydrogen bonding, to form transient nano-domains in membranes known as 'rafts' and on the surface of lipoprotein particles. Initially, there was a view that saturated sphingomyelin formed a liquid-ordered phase with cholesterol or a gel phase with saturated ceramides to lead to lateral segregation within the membrane, and that sphingomyelin and cholesterol metabolism were closely integrated, even that the sphingomyelin concentration might control the distribution of cholesterol in cells. On the other hand, the understanding of the mechanism of raft formation in membranes has changed substantially in recent years, and while an interaction with cholesterol is certainly important, it may not be the major factor in vivo. Ceramide can displace cholesterol from its association with sphingomyelin, when formed in membranes by hydrolysis of the latter.
Other functions: Sphingomyelin per se is generally considered to be a relatively inert molecule, although modern molecular biology methods are uncovering potential regulatory functions via interactions with particular proteins. For example, it has been shown to inhibit the activity of phospholipase A2α, a key enzyme in eicosanoid production. Sphingomyelin in the plasma membrane may be essential for the internalization of transferrin and thence of iron into cells, and it appears to be required for the activity of a number of membrane-bound proteins, including those of certain ion channels and receptors. As the most abundant sphingolipid in the nucleus, it is intimately involved in chromatin assembly and dynamics as well as being an integral component of the nuclear matrix. A single molecular species of sphingomyelin with a C18 acyl chain binds specifically to a coat protein designated 'p24' to enable it to form membrane vesicles. In addition, sphingomyelin is selectively recognized and acts as a receptor for the actinoporins, which are pore-forming toxins produced by sea anemones.
There is a specific binding site for sphingomyelin on the amyloid beta-peptide (Aβ) in brain, and there is evidence from studies in vitro that this may promote the aggregation of these proteins in Alzheimer's disease. In turn, this leads to depletion of brain sphingomyelin by activation of acid sphingomyelinase with disruption of many protein–lipid interactions and thence of downstream signaling pathways. In contrast, the ganglioside GM1 may have a protective role towards Aβ aggregation
As well as its role in membranes, it serves as a precursor for ceramides, long-chain bases, sphingosine-1-phosphate, and many other biologically important sphingolipids, as part of the 'sphingomyelin cycle' (also termed the ‘sphingolipid’ or ‘ceramide’ cycles depending on the context). Some of these metabolites are intra- and inter-cellular messengers, and others are essential membrane constituents. The sphingomyelin cycle extends to other sphingolipids via the action of sphingomyelinases and enzymes such as glycosylhydrolases and glycosyltransferases in cells to produce innumerable new oligoglycosylceramides. It can also give rise to sn-1,2-diacylglycerols, which are central to many metabolic and signaling pathways. These molecular relationships are illustrated only briefly in Figure \(\PageIndex{23}\).
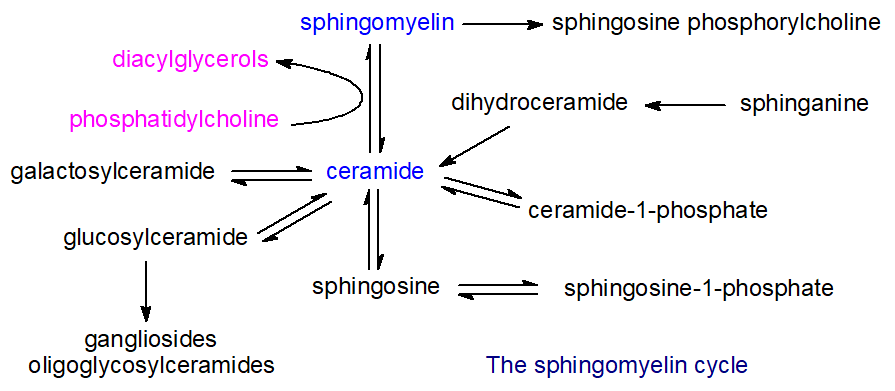
In particular, sphingomyelin is a major source of ceramides in most cellular organelles, including the nucleus and even mitochondria, via the action of sphingomyelinases (see next section), and in addition to being a source of other sphingolipids these are required to trigger apoptosis and other metabolic changes. As ceramides do not mix well with glycerophospholipids and cholesterol, this conversion results in the formation of new membrane domains enriched in ceramide that exclude cholesterol and so differ in composition from other sphingolipid rafts. This has profound effects on membrane function, especially of the plasma membrane, in that different proteins may be recruited or excluded depending on their relative affinities for cholesterol and ceramides. It may also influence disease states such as cancer.
Chlamydiae, widespread bacterial pathogens, acquire sphingomyelin from the Golgi apparatus and plasma membrane of their hosts and this is necessary for the viability and growth of the organisms. Other pathogenic bacteria, notably Pseudomonas aeruginosa and Neisseria gonorrhoeae, can hijack sphingomyelin catabolic enzymes with deleterious effects upon the host. Likewise, human immunodeficiency virus (HIV) and the hepatitis C virus utilize host sphingomyelin for their own nefarious purposes.
Nutrition: Although there is no known nutritional requirement for sphingomyelin and other sphingolipids, they are a component of any diet containing egg, meat or dairy products. Thus, it has been estimated that per capita sphingolipid consumption in the United States, for example, is of the order of 0.3-0.4 g/d. As sphingolipids constitute an appreciable proportion of the polar lipid constituents of milk, they may be significant if minor nutrients for infants and beneficial effects upon their development have been claimed. From animal experiments, there is evidence that dietary sphingolipids can reduce the intestinal absorption of cholesterol and other lipids, leading to reductions in serum lipid concentrations. Feeding sphingolipids inhibits colon carcinogenesis and may alleviate some of the symptoms of inflammatory bowel disease. 2-Hydroxyoleic acid suppresses the growth and induces autophagy in cancer cells by stimulating the synthesis of sphingomyelin and increasing the amount of this lipid in the plasma membrane. On the other hand, plasma sphingomyelin levels are considered to be an independent risk factor for atherosclerosis, possibly as a result of its ability to retain cholesterol in cells and the arterial wall with consequent diminished reverse cholesterol transfer via HDL.
Sphingomyelin Catabolism
In contrast to the glycerolipids, dietary sphingolipids are not hydrolyzed by pancreatic enzymes only. Rather, most of the sphingomyelin in the diet is hydrolyzed in the brush border of the intestines by an alkaline sphingomyelinase (at a pH of 8.5–9 optimally) to ceramide and thence by a neutral ceramidase to free fatty acids and sphingosine. Some of this enzyme is also present in liver from which it is secreted in bile into the intestinal lumen where it can hydrolyze sphingomyelin and other phospholipids with the aid of bile salts. The sphingosine released at the brush border is absorbed, some is re-N-acylated to form ceramides, and the remainder is converted via sphingosine-1-phosphate to palmitic acid, which is esterified into the triacylglycerol component of chylomicrons. In the process, some of these sphingolipid intermediates may have signaling functions and anti-inflammatory properties in intestinal cells. The alkaline sphingomyelinase is unusual in that is very different in its structure and other properties from intracellular enzymes with a related function; it is part of the (ecto)nucleotidepyrophosphatase-phosphodiesterase protein family (NPP) that includes autotaxin. The enzyme is believed to have a role in the production of sphingolipid metabolites within the intestines and colon especially, which may influence a number of disease states. For example, it appears to inhibit colon cancer by generating ceramides. In addition, alkaline sphingomyelinase has phospholipase C activity towards the pro-inflammatory metabolite platelet-activating factor and towards lysophosphatidylcholine with potentially further beneficial effects. By reducing the level of endogenous sphingomyelin and increasing that of ceramides in the membranes of intestinal cells, it is believed to reduce the uptake of dietary cholesterol.
Catabolism in other tissues: The key enzymes for the degradation of sphingomyelin to ceramides in most tissues are also sphingomyelinases (phosphodiesterases), as shown in Figure \(\PageIndex{24}\).
These are similar in function to phospholipase C and generate ceramides with their innumerable and important signaling properties as the main product. There are many such enzymes with different pH optima and metal ion requirements that operate in different regions of the cell with potentially distinct biochemical roles. Thus, there is an acid sphingomyelinase in the endo-lysosomes, and different neutral sphingomyelinases in the plasma membrane, endoplasmic reticulum, Golgi, and mitochondria in addition to the alkaline sphingomyelinase in the intestines. It should not be forgotten that the other product of the reaction is phosphocholine, which has importance as a nutrient. Bacterial sphingomyelinases are known to lyse red blood cells, although intriguingly, there is a sphingomyelinase in the bacterium Pseudomonas aeruginosa that can also act as a sphingomyelin synthase in vitro at least.
The lysosomal acid sphingomyelinase (pH optimum ca. 5) is expressed ubiquitously and has a key housekeeping role in maintaining normal membrane turnover and remodeling of the sphingolipid constituents, especially those of lipoproteins. While other lysosomal sphingolipid hydrolases require a saposin activator protein for full activity, the acid sphingomyelinase incorporates a built-in N-terminal saposin domain so does not require an external activator. Under resting conditions, acid sphingomyelinase is stored inside lysosomes, but upon stimulation, it undergoes vesicular transport to the plasma membrane where it docks with a specific protein and is exposed on the outer leaflet. It then generates ceramide by hydrolysis of sphingomyelin and initiates the train of events that leads to apoptosis. There are reports that acid sphingomyelinase, by acting at the plasma membrane to produce ceramides, regulates the localization and trafficking of palmitoylated proteins from the Golgi, and it may also facilitate bacteria-host interactions. Experiments in vitro have demonstrated that the enzyme can be considered as a phospholipase C that is active against a wide range of phospholipids, including ceramide-1-phosphate and the unique lysosomal phospholipid bis(monoacylglycero)phosphate.
There is a related secreted acid sphingomyelinase (Zn2+-dependent), which can be transported to the outer membrane of the cell and is especially important in endothelial cells of the human coronary artery. This enzyme is produced by the same gene but differs from the lysosomal enzyme as it requires Zn2+ ions for activation and has a different glycosylation pattern. It can also operate at neutral pH and has multiple functions in that it is involved in many aspects of cellular signaling as well as in membrane sphingomyelin turnover. By acting at the plasma membrane to produce ceramides, it is believed to regulate the trafficking of palmitoylated proteins from the Golgi to their new location.
Neutral sphingomyelinases (pH optima 7.4), of which four quite distinct enzymes are known, are located in membranes of the endoplasmic reticulum, Golgi, and plasma membrane with one in mitochondria (MA-NSM), where they have signaling functions by generating ceramides and thence other biologically active sphingolipids. Human NSM-1 has 423 amino acid residues and a molecular weight of 47.6 kDa; it has two putative transmembrane domains in the C-terminus and resides mainly in the nucleus and endoplasmic reticulum. It has a broad specificity for choline phospholipids, but it is most active with sphingomyelin and may not have a significant role in cellular signaling. In contrast, NSM-2 which is located in the Golgi apparatus and plasma membrane is activated by phosphatidylserine and is important for ceramide signaling. It is especially important in brain and nervous tissue, where it is required for the secretion of hypothalamic-sssreleasing hormones, although it is relevant to many cellular functions and physiological processes in most other tissues. Dysregulation of NMS-2 is reported to be a factor in many inflammation-related pathologies. Neutral sphingomyelinases-3 is found mainly in the plasma membrane of bone and cartilage, where it is vital for the process of mineralization; it is also important in striated and cardiac muscle. Little seems to be known of the function of the mitochondrial enzyme. Losses, mutation, and poor expression of the gene encoding neutral sphingomyelinase have been observed in several cancers, but exposure to ionizing irradiation led to rapid hydrolysis of sphingomyelin to ceramide by this enzyme, and thence to cancer cell death.
A diverse range of factors activates the enzymes, including chemotherapeutic agents, tumor necrosis factor-alpha, 1,25-dihydroxy-vitamin D3, endotoxin, gamma-interferon, interleukins, nerve growth factor, and most conditions known to induce cellular stress, especially in relation to inflammation. As they utilize by far the most abundant sphingolipid in animal tissues to generate ceramides and other sphingolipid metabolites that have important signaling functions, sphingomyelinases are believed to function as regulators of signaling mechanisms, especially in the nucleus of the cell. Thus, they have a much wider metabolic role than simply catabolism of sphingomyelin.
The type A and B forms of Niemann-Pick disease are lysosomal lipid storage disorders that are a consequence of a deficiency of acid sphingomyelinase with a resulting accumulation of sphingomyelin and smaller amounts of other sphingolipids, including gangliosides, in cells and tissues and especially in the monocyte/macrophage system to form the so-called “foam cells” that characterize the disease. A consequent lack of ceramide production may be involved in the pathology of the disease. Increasing sphingomyelin levels in turn result in elevated cholesterol concentrations. It is noteworthy that membranes containing ceramides have a much lower binding capacity for cholesterol, so sphingomyelin degradation may play a part in cholesterol homeostasis. Type C Niemann-Pick disease differs from the A and B forms and is caused by defects in two distinct cholesterol-binding proteins (NPC1 and NPC2).
Glucosyl- and Galactosylceramides (Cerebrosides)
There are two natural monoglycosylceramides of special importance in animals, i.e., glucosylceramide and galactosylceramide. Both have biological functions in their own right, but especially as structural components of membranes, as in the brain, for example, where galactosylceramide is required for the maintenance of the structure and stability of myelin and the differentiation of oligodendrocytes. Glucosylceramide is a vital component of all cell types, and is most abundant in human skin; it is the key intermediate in the biosynthesis of lactosylceramide and thence of complex oligoglycosphingolipids, including gangliosides. This monoglycosylceramide is also a major component of the membranes of plants and fungi. Although the two lipids have very similar structures in that D-galactose is an epimer of D-glucose and they differ only in the configuration at C4, they have very different biological properties. A few other monoglycosylceramides are produced in nature, for example by some bacteria of the order Sphingomonadales of α‑proteobacteria.
Structure and Occurrence
β-D-Galactosylceramide (Galβ1-1'Cer) is the principal glycosphingolipid in brain tissue, hence the trivial name "cerebroside", which was first conferred on it in 1874, although it was much later before it was properly characterized. In fact, galactosylceramides are found in all nervous tissues and indeed at low levels in all organs, but in they brain they can amount to 2% of the dry weight of grey matter and 12% of white matter or 23% of myelin lipids, where they insulate the axons of neuronal cells and constitute a substantial component of the extended plasma membrane of oligodendrocytes. It is also present in some fungal species. While galactosylceramide can be sulfated to form a sulfatide or sialylated to form ganglioside GM4, only a small proportion is subjected to further galactosylation to form Gal2Cer as the precursor for the limited gala-series of oligoglycosphingolipids.
β-D-Glucosylceramide (Glcβ1-1'Cer), with the trivial name "glucocerebroside", is a major constituent of skin lipids, where it is essential for the maintenance of the water permeability barrier of the skin. Otherwise, it is most abundant in animal tissues such as the spleen and erythrocytes as well as in nervous tissues, especially in the neurons if at low levels, and it is also found in plants. Higher than normal concentrations of this glycosphingolipid have been reported for the apical plasma membrane domain of epithelial cells from the intestines (especially the absorptive villous cells) and urinary bladder. The d18:1/16:0 molecular species of the two lipids are illustrated in Figure \(\PageIndex{25}\).
However, of equal or greater importance to the natural occurrence of glucosylceramide per se is its role as the biosynthetic precursor of lactosylceramide in animals, and thence of most of the complex neutral oligoglycolipids and gangliosides. In contrast, glucosylceramide is the end-product of the biosynthetic pathway in plants and fungi.
Interestingly, the proportion of galactosylceramides relative to glucosylceramides in myelin glycolipids increases greatly in the ascending phylogenic tree, and the ratio of hydroxy- to nonhydroxy fatty acids in cerebrosides increases with the complexity of the central nervous system. There is also an intriguing sex difference in the kidney, where it has been shown that galactosylceramide rather than glucosylceramide occurs in male mice only (or androgen-treated adult females). Only glucosylceramide is present in the nerves of the most primitive animals (protostomes).
In the brain, the galactosylceramides are enriched in very-long-chain fatty acids (C22–C26). The fatty acid and long-chain base compositions of cerebrosides from the intestines of the Japanese quail are listed in Table \(\PageIndex{5}\) for illustrative purposes. The fatty acid components resemble those of other sphingolipids, although the percentage of 2-hydroxy acids is higher than that in sphingomyelin, for example. They are exclusively saturated in this instance, though a small proportion of monoenoic components may also be found in other tissues. Glucosylceramides tend to contain mainly non-hydroxylated fatty acids that are of relatively shorter chain length. The proportion of trihydroxy bases listed is perhaps higher than in other many other tissues or species studied, probably reflecting the diet. Usually, sphingosine is the main long-chain base in cerebrosides of animal tissues.
| Table \(\PageIndex{5}\): Composition of fatty acids and long-chain bases (wt % of the total) in cerebrosides of intestines from the Japanese quail.* | ||||
| Long‑chain bases | Fatty acids | Non-hydroxy acids |
2-Hydroxy acids |
|
|---|---|---|---|---|
| Type | % | % | % | |
| t18:0 | 43 | 16:0 | 5 | 6 |
| d18:0 | 9 | 18:0 | 3 | trace |
| d18:1 | 27 | 20:0 | 2 | 4 |
| t20:0 | 6 | 21:0 | trace | 2 |
| d20:0 | 3 | 22:0 | 4 | 43 |
| d20:1 | 11 | 23:0 | 1 | 13 |
| 24:0 | 3 | 12 | ||
| * The cerebrosides comprised 81% galactosylceramide and 19% glucosylceramide. From Hirabayashi, Y. et al., Lipids, 21, 710-714 (1986); DOI. |
||||
Plants: Glucosylceramide is the only glycosphingolipid common to plants, fungi, and animals. It has often been described incorrectly as the main sphingolipid in plants, but this has been because the more polar complex glycosylinositol phosphoceramides are not easily extracted and until relatively recently were missed in conventional analyses. Nonetheless, glucosylceramide is abundant in photosynthetic tissues and constitutes approximately a third of the total sphingolipids, where the main long-chain bases are C18 4,8‑diunsaturated (Z/Z and E/Z) (not sphingosine as illustrated above); it is a major component of the outer layer of the plasma membrane and is also enriched in the late endosomes and plant tonoplast. Small amounts of monoglycosylceramides containing a β‑D‑mannopyranosyl unit may be present in non-photosynthetic tissues, but galactosylceramides have not been found in plants. Glucosylceramide is a common component of the lipids of yeast and other fungi, including most fungal pathogens. However, it does not occur in the yeast Saccharomyces cerevisiae, which is widely used as an experimental model, although trace levels of galactosylceramide have been detected.
The fatty acid and long-chain base compositions of glucosylceramides from two plant sources are listed in Table \(\PageIndex{6}\). Perhaps surprisingly, the fatty acid components are not very different in nature from those in animal tissues, comprising mainly longer-chain saturated and monoenoic acids, with a high proportion being saturated and having a hydroxyl group in position 2. In the examples selected for the table here, both di- and tri-hydroxy long-chain bases were found, mainly diunsaturated (Z/Z and E/Z) and almost entirely C18 in chain length. Much higher concentrations of glucosylceramides are found in pollen than in leaves, with substantial compositional differences. For example, the long-chain bases in Arabidopsis leaves consist mainly of t18:1, with relatively little d18:1, t18:0 and d18:0 (with 16:0, 24:0 and 24:1 hydroxy fatty acids mainly), but no d18:2 base although this is 50% of those in pollen. While saturated 2-hydroxy acids predominate in most plants, some cereal glucosylceramides contain high proportions of mono-unsaturated very-long-chain fatty acids of the n-9 family. Glucosylceramides from algae tend to resemble those from higher plants, although some novel structures have been reported from microalgae.
| Table \(\PageIndex{6}\): Composition of fatty acids and long-chain bases (wt % of the total) in glucosylceramides of seeds from scarlet runner beans and kidney beans. | |||||
| Fatty acidsa | Long-chain basesb | ||||
|---|---|---|---|---|---|
| Type | Runner beans | Kidney beans | Runner beans | Kidney beans | |
| % | % | % | % | ||
| 16:0 | 4 | 5 | t18:0 | trace | trace |
| Other non-hydroxy |
1 | 2 | t18:1-8t | 13 | 11 |
| 14:0-OH | 1 | 1 | t18:1-8c | 10 | 9 |
| 15:0-OH | 1 | 1 | d18:0 | trace | trace |
| 16:0-OH | 58 | 58 | d18:1-8c/t | 1 | 3 |
| 18:0-OH | trace | trace | d18:1-4t | trace | trace |
| 20:0-OH | trace | trace | d18:2-4t,8t | 45 | 60 |
| 22:0-OH | 7 | 6 | d18:2-4t,8c | 31 | 17 |
| 23:0-OH | 2 | 1 | |||
| 24:0-OH | 23 | 23 | |||
| 25:0-OH | 1 | 1 | |||
| 26:0-OH | 1 | 1 | |||
| From Kojima, M. et al., J. Agric. Food. Chem., 39, 1709-1714 (1991); DOI, but see also Yamashita, S. et al. for further data: DOI a including 2-hydroxy acids; b di- and tri-hydroxy bases with cis or trans double bonds in the positions indicated. |
|||||
Biosynthesis
Ceramides synthesized both de novo and by catabolism of sphingomyelin are used for the biosynthesis of monoglycosylceramides in animal tissues. The biosynthetic mechanism resembles that for glycosyldiacylglycerols, i.e., there is a direct transfer of the carbohydrate moiety from a sugar-nucleotide, e.g. uridine 5-diphosphate(UDP)-galactose, UDP-glucose, etc, to a ceramide unit synthesized in the endoplasmic reticulum. This is illustrated in Figure \(\PageIndex{26}\).
During the transfer, which is catalyzed by specific glycosyl-transferases, inversion of the glycosidic bond occurs from the alpha to beta configuration. Synthesis of β-D-galactosylceramide takes place on the lumenal surface of the endoplasmic reticulum, although it has free access to the cytosolic surface by an energy-independent flip-flop process. Expression of the UDP-galactose:ceramide galactosyl transferase (galactosylceramide synthase) is restricted to oligodendrocytes, Schwann cells, kidneys, and testes. Prior to sulfation, galactosylceramide is transported to the trans-Golgi compartment.
In contrast, after the transfer of the precursor ceramides from the endoplasmic reticulum to the cytosolic side of the early Golgi membranes with the aid of the CERT protein, glucosylceramide is produced by a glucosylceramide synthase present in this membrane (with the possible exception of neuronal tissues). If it is to be converted to more complex oligoglycosylceramides, this must be translocated to the luminal leaflet of the trans-Golgi membranes, a process that occurs both by vesicular and by non-vesicular transport. The latter is mediated by a conserved clade of integral membrane proteins, i.e., phospholipid flippases (P4-ATPases) designated ATP10A and ATP10D, together with the four phosphate adapter protein-2 (FAPP2) and glycolipid transfer protein (GLTP) in humans with related enzymes in fungi, which utilize the energy from ATP catalysis to translocate lipids across cellular membranes. The human enzymes are entirely specific for glucosylceramide and not galactosylceramide. Indeed, the galactosyl- and glycosylceramide synthases have no significant sequence homology, indicating different evolutionary origins.
For their functions in protein interactions and signaling, both galactosyl- and glucosylceramide must be transported to and then across the plasma membrane. Some glucosylceramide is carried by lipoproteins (VLDL, LDL, and HDL) in the circulation and presumably requires active transport for absorption and distribution across the membranes of target tissues.
In plants, glucosylceramides are also formed by an evolutionarily conserved glucosylceramide synthase involving UDP-glucose in the endoplasmic reticulum, although an alternative mechanism has been described that utilizes sterol glucoside as the immediate glucose donor to ceramide. There is also evidence for a requirement for ceramides containing Δ4 trans-double bonds for synthesis of glucosylceramides but not other sphingolipids in some plant and fungal tissues. However, there is a distinct ceramide synthase in the yeast Pichia pastoris, which produces ceramides of defined composition exclusively for the production of glucosylceramides. A separate ceramide synthase with different specificities produces the ceramide precursors for ceramide phosphorylinositol, which contains only phytosphingosine as the long-chain base. In fungi, glucosylceramide synthases have been characterized, but a galactosylceramide synthase has yet to be identified. Enzymes responsible for the biosynthesis of glucuronosylceramide and α-galactosylceramide in some bacterial species have been characterized.
Function
Galactosylceramides: A remarkable property of cerebrosides is that their 'melting point' is well above physiological body temperature, so that glycolipids have a para-crystalline structure at this temperature. Each cerebroside molecule may form up to eight inter- or intramolecular hydrogen bonds by lateral interaction between the polar hydrogens of the sugar and the hydroxy and amide groups of the sphingosine base of the ceramide moiety, and this dense network of hydrogen bonds is believed to contribute to the high transition temperature and the compact alignment of cerebrosides in membranes. As with sphingomyelin, monoglycosylceramides tend to be concentrated in the outer leaflet of the plasma membrane together with cholesterol and thence in myelin in the specific membrane domains termed 'rafts'. Indeed, the latter appear to facilitate segregation to a greater extent than sphingomyelin via the combination of hydrogen bonds and hydrophobic interactions, and these forces are also of great importance for binding to the wide range of proteins, including enzymes and receptors, which are found in raft domains.
Galactosylceramide is essential to myelin structure and function and it is involved in oligodendrocytes differentiation. While molecular species with 2’-hydroxy fatty acid constituents are not essential for myelin formation, they are critical for the long-term stability of myelin, presumably because increased hydrogen bonding with neighboring lipids in membranes stabilizes the phase structure. Galactosylceramide is important as a precursor of 3’-sulfo-galactosylceramide, which is also essential to brain development in addition to numerous functions in other tissues. By interacting with sulfatide located in the membrane of opposing layers in the myelin sheath by carbohydrate-carbohydrate interaction, it forms what is known as a glycosynapse, which provides a necessary contribution to the long-term stability of myelin.
Glucosylceramides: Glucosylceramides have similar physical properties in membranes to the galactose analog, and they are also concentrated in raft domains in the outer leaflet of the plasma membrane. As mentioned briefly above, they are the primary precursor for most of the more complex oligoglycosphingolipids in animal tissues, especially in brain, where synthesis is vital for the production of most neuronal oligoglycosphingolipids, while glucosylceramide per se is essential for axonal growth. They are major constituents of skin lipids, where they are essential for lamellar body formation in the stratum corneum and to maintain the water permeability barrier of the skin. In addition, the epidermal glucosylceramides (together with sphingomyelin) are the source of the unusual complex ceramides that are found in the stratum corneum including those with terminal hydroxyl groups and estolide-linked fatty acids. Some of the glucosylceramide in the skin is linked covalently to proteins via terminal hydroxyl groups, presumably to strengthen the epidermal barrier.
Much of the evidence for the function of glycosylceramides in animals has been derived from cell lines in which synthesis of the lipid has been suppressed by various means in vitro. It appears that glucosylceramide is not essential for the viability of certain cell lines in culture, but disruption of the global synthase gene in mice results in the death of embryos. It is essential for the survival of cancer cells, and deletion from other cell types can lead to abnormalities. In addition to being an intermediate in the biosynthesis of more complex glycosphingolipids and its role in the permeability barrier of the skin (discussed above), glucosylceramide is believed to be required for intracellular membrane transport, cell proliferation, and survival, and for various functions in the immune system. In contrast, there are indications that it may have adverse implications for various disease states. For example, over-expression of glucosylceramide synthase in cancer cells has been linked to tumour progression with a reduction in ceramide concentration, resulting in increased resistance to chemotherapy. The lipid has also been associated with drug resistance in a wider context. In the nematode Caenorhabditis elegans, glucosylceramide containing the fatty acid 22:0 is reported to be a longevity metabolite that functions through the membrane localization of clathrin, a protein that regulates membrane budding.
In Arabidopsis, glucosylceramides are critical for cell differentiation and organogenesis, but not necessarily for the viability of cells. It has been proposed that glycosphingolipids could impose positive curvature to membranes, thereby facilitating vesicle fusion. There is evidence that glycosylceramides (but not glycosyldiacylglycerols) together with sterols are located in 'rafts' in plant membranes in an analogous manner to sphingolipids in animal tissues, and that they are associated with specific proteins. Correlative studies suggest that glucosylceramides help the plasma membrane in plants to withstand stresses brought about by cold and drought. For example, glycosylceramides containing 2-hydroxy monounsaturated very-long-chain fatty acids and long-chain bases with 4-cis double bonds appear to be present in higher concentrations in plants that are more tolerant of chilling and freezing. While fungal glucosylceramides with a 9-methyl group within the sphingosine backbone elicit defence responses in rice, cerebrosides with double bonds in positions 4 and/or 8 of the long-chain base appear to be involved in the defense of some plant species against fungal attack.
Less is known of the function of glucosylceramides in fungi, although they are certainly major constituents of the plasma membrane and cell wall. They are believed to be involved in such processes as cell wall assembly, cell division and differentiation, and signaling. The presence of the methyl branch in the long-chain base is essential for cell division and alkali tolerance. In the case of fungal pathogens, glucosylceramides are recognized by the host immune system and regulate virulence, often after export into the external environment as extracellular vesicles. In contrast to animals, ceramide monohexosides are not precursors for oligoglycosylceramides in fungi. Some molecular species of this lipid from plants (a Δ8 double bond in the long-chain base is essential) show fruiting-inducing activity in the fungus Schizophyllum commune.
α-D-Galactosylceramides: Cerebrosides linked to an α-D- rather than a β-D-galactosyl unit such as that found in the marine sponge Agelas mauritianus, in human gut microflora, and even in cow's milk are potent stimulators of mammalian immune systems by binding to the protein CD1d on the surface of antigen-presenting cells and activating invariant natural killer T cells. Indeed this was one of the first pieces of evidence to show that glycolipids, like glycoproteins, could invoke an immune response. Subsequently, it was demonstrated that α-galactosylceramide with a 24:1 fatty acid, though present in very small amounts, is loaded onto the CD1d or CD40 protein and is presented as the natural endogenous ligand for NKT cells in the thymus and the periphery. Once activated, NKT cells secrete a range of pro-and anti-inflammatory cytokines to modulate innate and adaptive immune responses. The α‑glucosyl and α‑psychosine analogs show similar activity.
It is not certain whether α-galactosylceramide is synthesized in animal tissues, and it is likely that is derived primarily from members of the gut microbiome such as Bacteroides fragilis and related species (although in general, few bacterial species produce sphingolipids). Ceramide-galactosyltransferases responsible for the synthesis of this lipid in two species of bacteria from the intestinal microbiome have been identified. In mouse gut, the main molecular form consisted of a 2‑(R)‑hydroxylated hexadecanoyl chain linked to C18-sphinganine, while that in B. fragilis contained longer-chain components with iso-methyl-branches in the sphingoid base and often fatty acid moieties. The sphinganine chain branching is a critical determinant of NKT cell activation by the bacterial enzyme. A decrease in the production of this lipid was observed in mice exposed to stress conditions that alter the composition of the gut microbiota, including Western-type diet, colitis, and influenza A virus infection with potential consequences upon the systemic immune responses. Its concentration within animal tissues is controlled by catabolic enzymes in a two-step mechanism: removal of the acyl chain by an acid ceramidase followed by hydrolysis of the sugar residue by an α-glycosidase. Initial studies with animal models suggest that treatment with α‑D‑galactosylceramides is effective against lung and colorectal cancers, melanomas and leukemia, and pre-clinical trials of this lipid and synthetic analogs so far have shown that these are safe and effective as an anti-tumour immunotherapeutic agents and vaccine adjuvants. Indeed, a phase I trial with high-risk melanoma patients has given promising preliminary results.
Catabolism of Glycosphingolipids
In animal tissues, the main sites for the degradation of all glycosphingolipids, including the monoglycosylceramides, oligoglycosphingolipids and gangliosides, are the lysosomes. These are membrane-bound organelles that comprise a limiting external membrane and internal lysosomal vesicles, which contain soluble digestive enzymes that are active at the acidic pH of this organelle. All membrane components are actively transported to the lysosomes to be broken down into their various primary components. In the case of glycosphingolipids, this means to fatty acids, sphingoid bases, and monosaccharides, which can be recovered for re-use or further degraded. Thus, sections of the plasma membrane enter the cell by a process of endocytosis, and they are then transported through the endosomal compartment to the lysosomes. The compositional and physical arrangement of the lysosomal membranes is such that they are themselves resistant to digestion with bis(monoacylglycero)phosphate (lysobisphosphatidic acid) as a characteristic component of the inner membrane. A glycocalyx of highly N-glycosylated integral membrane proteins protects the perimeter membrane with the aid of the ganglioside GM3, which is resistant to degradation. This glycocalix forms an efficient hydrophilic barrier at the luminal surface of the lysosomal perimeter membrane to protect it from degradation by proteases and hydrolases, and to prevent lipids and their hydrolysis products from escaping from the lumen of the lysosome.
Degradation of oligoglycosylceramides and gangliosides occurs by sequential removal of monosaccharide units via the action of specific exohydrolases from the non-reducing end until a monoglycosylceramide unit is reached when glucosylceramide β-glucosidases or an analogous β-galactosidase (one isoform) removes the final carbohydrate moiety. Several glucosylceramidases are known; GBA1 is a lysosomal hydrolase, GBA2 is a ubiquitous non-lysosomal enzyme and GBA3 is a cytosolic β-glucosidase. The last is found in the kidney, liver, spleen and a few other tissues of mammals, but its function is not clear.
As glycolipids with fewer than four carbohydrate residues are embedded in intralysosomal membranes, while the degradative enzymes are soluble, the process requires the presence of negatively charged lipids and specific activator proteins, which are water-soluble glycoproteins of low molecular weight. These are not themselves active catalytically but are required as cofactors either by directing the enzyme to the substrate or by activating the enzyme by binding to it in some manner. Five such proteins are known, the GM2-activator protein (specific for gangliosides) and Sphingolipid Activator Proteins or saposins A, B, C and D, which perturb the membranes sufficiently to enable the degradative enzymes to reach the glycolipid substrates. The four saposins are derived by proteolytic processing from a single precursor protein, prosaposin, which is synthesized in the endoplasmic reticulum, transported to the Golgi for glycosylation, and then to the lysosomes. Of these, saposin C is essential for the degradation of galactosyl- and glucosylceramide, while saposin B is required for the hydrolysis of sulfatide, globotriaosylceramide, and digalactosylceramide. The products of the hydrolysis reaction with monoglycosylceramides are ceramides and monosaccharides with net retention of the stereochemistry of the latter in the process. This is illustrated in Figure \(\PageIndex{26}\).
The reactions are aided by the presence of anionic lipids such as bis(monoacylglycero)phosphate. In particular, this increases the ability of the GM2-activator to solubilize lipids and stimulates the hydrolysis of membrane-bound GM1, GM2, and some of the kidney sulfatides. Saposin D stimulatesthe degradation of lysosomal ceramide by acid ceramidase, and it is also involved in the solubilization of negatively charged lipids at an appropriate pH. Eventually, the ceramides can in turn be hydrolyzed by an acid ceramidase to fatty acids and sphingoid bases.
β-Glucosylceramidase and saposin C are also required for the generation of the structural ceramides from glucosylceramide in the outer region of the skin, a process essential for optimal skin barrier function and survival. Some glucosylceramide is hydrolyzed by the enzyme GBA2 at the plasma membrane, where the ceramide formed is rapidly converted to sphingomyelin by the sphingomyelinase 2, which may be co-located with the glucosidase. In addition, it has been established that cellular β-glucosidases are able to transfer the glucose moiety from glucosylceramide to and from other lipids as in the formation of cholesterol glucoside.
Small but significant amounts of glucosyl- and galactosylceramides are ingested as part of the human diet. They are not hydrolyzed by pancreatic enzymes but are degraded in the brush border of the intestines by the enzyme lactase-phlorizin hydrolase (which also hydrolyses the lactose in milk) to ceramides and thence to sphingosine.
An Arabidopsis homolog of human glucosylceramidase (AtGCD3) preferentially hydrolyses glucosylceramides that contain long acyl chains, and three further isoforms may exist based on sequence homology.
Genetic disorders and Disease
Harmful quantities of glucosylceramide accumulate in the spleen, liver, lungs, bone marrow, and, in rare cases, the brain of patients with Gaucher disease, the most common of the inherited metabolic disorders (autosomal recessive) involving storage of excessive amounts of complex sphingolipids. Three clinical forms (phenotypes) of the disease are commonly recognized of which by far the most dangerous are those affecting the brain (Types 2 and 3). All of the patients exhibit a deficiency in the activity of the lysosomal glucosylceramide-β-glucosidase (GBA1), which catalyzes the first step in the catabolism of glucosylceramide. The enzyme may be present, but a mutation prevents it from forming its correct conformation, although other factors may be involved as patients with a defective saposin C, the lysosomal activator protein, develop similar symptoms.
In the brain, glucosylceramide accumulates when complex lipids turn over during brain development and during the formation of the myelin sheath of nerves. Other than in the brain, the excess glucosylceramide arises mainly from the biodegradation of old red and white blood cells. The result is that the glucosylceramide remains stored within the lysosomes of macrophages, i.e., the specialized cells that remove worn-out cells by degrading them to simple molecules for recycling, thus preventing them from functioning normally and often leading to chronic inflammation. The enlarged macrophages containing undigested glucosylceramide are termed Gaucher cells. They over-express and secrete certain proteins into the circulation, and some of these are used as biomarkers. In addition, glucosylceramide is converted more rapidly to gangliosides in these cells, leading to an increase in ganglioside GM3 in the plasma and spleen of patients with Gaucher disease. Fortunately, there are now effective enzyme replacement therapies for patients with the milder (non-neurological or Type 1) form of Gaucher disease that successfully reverse most manifestations of the disorder, including decreasing liver and spleen size and reducing skeletal abnormalities. Two oral drugs that inhibit glucosylceramide synthesis have also been approved.
Defective GBA1 enzyme activity in humans has been implicated in an increased risk of multiple myeloma and other cancers. Oligoglycosylceramides and gangliosides in particular are known to be involved in the pathology of a number of cancers, and glucosylceramide is an important precursor of these. Inhibition of glucosylceramide synthase, which is overexpressed in many human tumors lead to a marked arrest of cell growth in cancer cells in vitro, so this is believed to have the potential for the treatment of colorectal and other cancers.
A deficiency in glucocerebrosidase activity may predispose individuals to more common disorders such as Parkinson's disease and Lewy body dementia. Excess glucosylceramide production and thence of more complex glycosphingolipids is a factor in polycystic kidney disease. It appears to be a general rule that the mere process of lysosomal substrate accumulation in all lysosomal storage disorders impairs lysosome integrity and results in more general disruptions to lipid metabolism and membrane structure and function. On the other hand, inhibition of glucosylceramidases may be of benefit in cystic fibrosis. Krabbe disease is discussed in the next section.
Galactosylceramide is believed to function as an initial receptor for the human immunodeficiency virus (HIV) in mucosal epithelial cells and controls the early infection-independent phase of HIV transfer to T cells. Glucosylceramide levels regulate the uptake of viruses that rely upon the late endosomal compartment for fusion, including the influenza A and Ebola viruses.
Gangliosides
The name ganglioside was first applied by the German scientist Ernst Klenk in 1942 to a mixture of complex glycosphingolipids newly isolated from ganglion cells of brain. Subsequently, he demonstrated that as part of an oligosaccharide chain, they contained an acidic carbohydrate component, which he named "neuraminic acid" - later termed "sialic acid" from the Greek "sialon" for saliva, from which they were first isolated. However, it was not until 1963 that the first ganglioside species was fully characterized. Innumerable sphingolipids are now known that differ in the nature of both the glycan (glucose, galactose, N-acetylgalactosamine, and sialic acid residues) and ceramide structures. They are present throughout the animal kingdom, from echinoderms up to higher animals, but not in plants. Such highly polar, acidic and relatively hydrophilic molecules have distinctive physical properties, which are essential for the vital functions of gangliosides in the membranes of the central nervous system and other tissues.
Sialic acids and Gangliosides
Sialic acids: Gangliosides are oligoglycosylceramides derived as a first step from lactosylceramide, and they are defined by the presence of one to as many as five sialic acid residues, i.e. carbohydrate molecules with a nine-carbon backbone and a carboxylic acid group, a subclass of the superfamily of naturally occurring non‑2‑ulosonic acids. Of the many forms that have been characterized, only a few are relevant to gangliosides, and the most important of these is N-acetylneuraminic acid (‘NANA’ or ‘SA’ or 'Neu5Ac' or 'NeuAc'). Less often the sialic acid component is N-glycolylneuraminic acid (Neu5Gc), which differs by only one oxygen atom at the C-5 N-acetyl group, or it can be a Neu5Ac analogue in which the amide group is replaced by a hydroxyl group, i.e. 3-deoxy-D-glycero-D-galacto-nonulosonic acid (ketodeoxynonulosonic acid or ‘KDN’). The sialic acids are joined via α-glycosidic linkages to one or more of the monosaccharide units, e.g. via the hydroxyl group on position 2, or to another sialic acid residue. The polar head groups of the lipids carry a net-negative charge at pH 7.0 and they are acidic. Their structures are shown in Figure \(\PageIndex{27}\).
Humans lack Neu5Gc: Neu5Ac is the biosynthetic precursor of Neu5Gc, a component of gangliosides from most animal species, including mice, horse, sheep, and goats, via the action of the enzyme CMP–N-acetylneuraminic acid hydroxylase (CMAH). However, NeuGc is not synthesized in humans (or birds and New World monkeys), although it is present in other primates such as the great apes, and indeed as it is a xeno-antigen, anti-NeuGc antibodies are produced normally in healthy humans (and especially after injection of NeuGc-containing glycoconjugates). The absence or irreversible inactivation of a number of relevant genes, but especially a critical exon in the CMAH gene, both for sialolipids and peptides in humans suggests that this may have been a major biochemical branch-point in human evolution that occurred ~2 to 3 million years ago after the divergence of humans and chimpanzees from a common ancestor. It may even be a factor in the superior performance of the human brain as the overexpression of Neu5Gc in the brains of transgenic mice was found to result in abnormal development. It could also mean that there might have been a fertility barrier between us and other hominids during evolution.
While these are speculations, there is some evidence that the loss of Neu5Gc in humans had complex effects on immunity, providing greater capabilities to clear sublethal bacterial challenges. Some NeuGc may be obtained from the diet in meat and milk, for example, and this may be incorporated into human gangliosides to a limited extent, especially in fetal tissues and some cancers. In the latter, preferential expression of dietary Neu5Gc has been ascribed to their higher metabolic rate.
2. Structure and Occurrence of Gangliosides
Most of the common range of gangliosides are derived from the ganglio- and neolacto-series of neutral oligoglycosphingolipids (Table 1), and they should be named systematically in the same way with the position of the sialic acid residue(s) indicated as for branched structures. However, they are more conveniently defined by a short-hand nomenclature system proposed by Svennerholm in which M, D, T and Q refer to mono-, di-, tri- and tetrasialogangliosides, respectively, and the numbers 1, 2, 3, etc refer to the order of migration of the gangliosides on thin-layer chromatography. For example, the order of migration of monosialogangliosides is GM3 > GM2 > GM1 (sometimes defined by subscripts, e.g. GM1 or GM1). To indicate variations within the basic structures, further terms are added, e.g. GM1a, GD1b, etc. Although alternatives have been proposed that are more systematic in structural terms, the Svennerholm nomenclature is that approved by IUPAC-IUB. Ganglio-series glycosphingolipids having 0, 1, 2 and 3 sialic acid residues linked to the inner galactose unit are termed asialo- (or 0-), a-, b- and c-series gangliosides, respectively, while gangliosides having sialic acid residues linked to the inner N-galactosamine residue are classified as α-series gangliosides. The structures for these groups are illustrated in the section on ganglioside biosynthesis below, for reasons of practical convenience.
As of 2020, more than 200 gangliosides with variations in the carbohydrate chain had been characterized in vertebrates alone. One of the most studied monosialo-gangliosides and the first to be fully characterized is ganglioside GM1a (Neu5Acα2-3(Galβ1-3GalNAcβ1-4)Galβ1-4Glcβ1Cer), a major brain ganglioside of mammals and the preferred ligand of cholera toxin, illustrated in Figure \(\PageIndex{28}\).
It can also be depicted using the abbreviated structure shown in Figure \(\PageIndex{29}\).

An alternative nomenclature, which is less used, is recommended by IUPAC-IUB and is based upon the ganglio (Gg) root structure; it employs Roman numerals to designate each hexose unit and the location of the Neu5Ac along the carbohydrate chain with Arabic superscripts to designate the hydroxyl group to which this is linked. By this system, GM1a is defined as II3-α-Neu5Ac-Gg4Cer.
Brain gangliosides: Gangliosides can amount to 6% of the weight of lipids from the brain (20 to 500 times more than in other tissues), where they constitute 10 to 12% of the total lipid content (20-25% of the outer layer) of neuronal membranes, for example. Aside from this, they are synthesized and are present at low levels (1 to 2% of the total lipids) in all animal tissues, where like the neutral oligoglycosphingolipids they are concentrated in the outer leaflet of the plasma membrane in the nanodomains known as 'rafts' or in related structures. Mammalian neurons actively synthesize gangliosides of the ganglio-series primarily, but oligodendrocytes in the brain produce instead myelin-forming glycosphingolipids, such as galactosylceramide and sulfatide together with a minor amount of ganglioside GM4.
The brain contains as much as 20 to 500 times more gangliosides than most non-neural tissues, with three times as much in grey as in white matter. As the brain develops, there is an increase in the content of gangliosides and in their degree of sialylation. There are large differences between species and tissues. For example, during embryogenesis and the postnatal period in the human central nervous system, the total amount of gangliosides increases approximately threefold, while that of GM1 and GD1a increases 12 to 15-fold. During the same period, the hemato-series gangliosides GM3, GD3, and 9-OAc-GD3, which lack a hexosamine residue, are the predominant ganglioside species, but they are present in much lower amounts in adults and then in some areas of the brain only. In the mouse brain, the total amount of gangliosides is almost 8-fold greater in adults than in embryos, with a similar shift in composition from simple (GM3 and GD3) to more complex gangliosides. It is evident that the ganglioside changes during brain maturation are correlated with many neuro-developmental milestones, and there is no doubt that gangliosides play a crucial role in neuronal function and brain development, especially during infancy when there is high nutrient demand as the brain undergoes rapid restructuring.
The main gangliosides (~95%) of adult mammalian brain are ganglio series GM1, GD1a, GD1b, and GQ1b, while lactosyl series gangliosides such as GM3 (sialyllactosylceramide) are found mainly in the extra-neural tissues. The remaining ~5% consists of minor components in the brain include gangliosides GM4, GM3, GD3, GM2, GD2, Fuc-GM1, Fuc-GD1b, GT1a and GP1c, the proportions of which vary depending on species. On the other hand, modern mass spectrometric methodology (electrospray ionization ion mobility MS) has revealed a much higher degree of sialylation than was previously recognized, including a complete series of mono- to octasialylated gangliosides in fetal frontal lobe. Subsequently, many previously unknown acetylated gangliosides were found in fetal hippocampus by this methodology. The content and composition of gangliosides in the brain also change with aging, with a substantial fall in the content of lipid-bound sialic acid but an increase in the proportion of the more complex forms in terms of carbohydrate structures in the elderly.
Gangliosides in other tissues and species: Among the extraneural tissues, lactosyl series gangliosides such as GM3 (sialyllactosylceramide) and monosialogangliosides, in general, tend to predominate. Relatively high concentrations of ganglioside GD1a are present in erythrocytes, bone marrow, testis, spleen, and liver, while GM4 is more abundant in kidney, GM2 in bone marrow, GM1 in erythrocytes and GM3 in intestine. In germ cells of mice, there is a switch between gangliosides of the a- and 0-series upon differentiation when they are crossing the blood-testis barrier. Skin fibroblasts and many cells of visceral organs generate gangliosides of the globo series mainly. Similarly, glob-o and lacto series gangliosides are characteristic components of the stage-specific embryonic antigens (SSEA), which underlie the development and differentiation of human embryonic stem cells. A sialyl-lactotetraosylceramide is present in the latter and in the brains of children under the age of two, but not in tissues of adult humans. Gangliosides can cross the placental barrier into the fetus and those in milk, derived from the apical plasma membrane of secretory cells of the mammary gland, may be of nutritional importance for the newborn. GD3 is the main ganglioside in human breast milk at an early stage of lactation, whereas GM3 is more abundant in the later stages (and in bovine milk). Unfortunately, gangliosides are poorly characterized and quantified in foods in general.
A 5-N-deacetylated form of ganglioside GM3 has been detected in human melanoma tumors. In addition, O-acetylation or lactonization of the sialic acid residue adds to the potential complexity. Gangliosides containing O-acetylated sialic acids, such as 9-OAc-GD3, are expressed during embryonic development and in the retina and cerebellum of adult rats, but not other brain regions. They occur also in certain tumors and may protect them from apoptosis. It is possible that such gangliosides are even more widespread, but they are missed after treatment with mild alkali during the isolation procedure, a common analytical practice. A further complexity is the occurrence of gangliosides with sulfate groups, and these have been isolated from human, mouse, and monkey kidney cells. KDN-containing gangliosides are minor components of egg, ovarian fluid, sperm and testis of fish and of some mammalian tissues
Gangliosides from marine invertebrates (echinoderms), such as starfish and sea cucumbers, are very different in structure from those in vertebrates and do not have a shorthand nomenclature. They include forms with distinctive ceramide compositions, untypical carbohydrate residues, sialic acids within the oligosaccharide chain, or with glycosyl inositol-phosphoceramide structures. The mollusc, Aplysia kurodai, lacks gangliosides but produces complex oligoglycosylceramides with 2-aminoethylphosphonic acids and/or phosphoethanolamine groups attached that may serve as ganglioside surrogates.
Ceramide structures: In general, the ceramide structures of gangliosides tend to be relatively simple. Sphingosine is usually the main sphingoid base, accompanied by the C20 analog in gangliosides of the central nervous system. Stearic acid (18:0) can be 80 to 90% of the fatty acid constituents in the brain, accompanied by small amounts of 16:0, 20:0 and 22:0, but with little or no polyunsaturated or 2-hydroxy acids, other than in some exceptional circumstances (e.g. some carcinomas). Palmitic acid is more abundant in gangliosides of the intestines and liver, while 2-hydroxylated fatty acids are relatively abundant in the last and in the kidney. There are also differences in the composition of the base and fatty acid components in different cells or regions of the brain. During development, the nature and concentrations of these constituents change markedly, and for example, the ratio of C20/C18-sphingosine in ganglioside GD1a of cerebellum increases 16-fold from 8-day-old to 2-year-old rats. In gangliosides outwith the nervous system, C20-sphingosine is barely detectable, and there is often a much wider range of fatty acid constituents (C14 to C24).
The nature of the ceramide component is relevant to the biological function of gangliosides, and changing the fatty acid component to α-linolenic acid by synthetic means alters the biological activity of gangliosides dramatically in vitro. However, it is the carbohydrate moiety that has the primary importance for most of their functions, and detailed discussion of these structures would take us into realms of chemistry best left to carbohydrate experts (see the reading list below). In any given cell type, the number of different gangliosides may be relatively small, but their nature and compositions may be characteristic and in some way related to the function of the cell. It is noteworthy that some terminal glycan structures of gangliosides are also present in glycoproteins of membranes.
3. Biosynthesis
There is evidence that the pool of glucosylceramide and thence of lactosylceramide that is utilized for ganglioside biosynthesis is different from that for the other neutral oligoglycosylceramides. This may explain some of the differences between the two groups in the fatty acid and sphingoid base components, which will also be dependent upon cell type. It is an open question how the ganglioside precursors enter the Golgi and trans-Golgi network where synthesis occurs at the luminal leaflet, but it appears that the regulation of intracellular sphingolipid traffic may be as important as the control of enzyme expression and activity in determining the final compositions of the various glycosphingolipid types.
In humans, sialic acid biosynthesis occurs by a series of reactions in the cytosol, but the Neu5Ac produced is transferred to the nucleus and activated by the cytosine 5'-monophosphate N-acetylneuraminic acid synthetase (CMAS) to form CMP-Neu5Ac, which is transported to the Golgi apparatus by a family of sialyltransferases specific for particular glycosidic linkages (α2,3, α2,6, α2,8, and α2,9).
Thereafter, the pathways for the biosynthesis of the common series of gangliosides of the ganglio-series, for example, involve sequential activities of distinct membrane-spanning sialyltransferases and glycosyltransferases as illustrated in Figure \(\PageIndex{29}\) for the four main 0-, a-, b- and c-series of gangliosides.
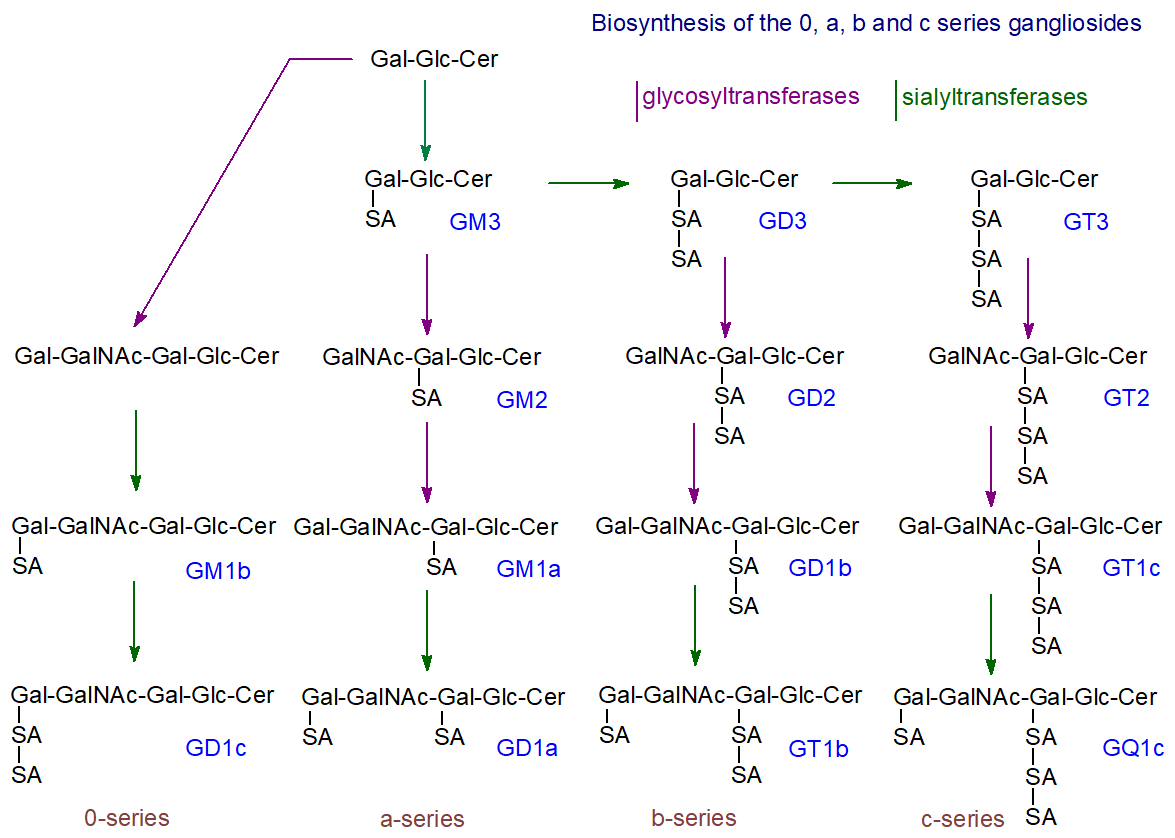
The required enzymes are bound to the membranes of the Golgi apparatus in a sequence that corresponds to the order of addition of the various carbohydrate components. Thus, the sialyltransferase that catalyzes the synthesis of the relatively simple ganglioside GM3 is located in the cis-region of the Golgi, while those that catalyse the terminal steps of ganglioside synthesis are located in the distal or trans-Golgi region. The GM3 synthase in particular, which catalyzes the transfer of Neu5Ac from cytidine monophosphate (CMP)-Neu5Ac onto the terminal galactose residue of lactosylceramide, has a unique specificity.
The simple ganglioside GM3 is synthesized by the addition of sialic acid to lactosylceramide by CMP:LacCer α2-3 sialyltransferase (or GM3 synthase), before GD3 and GT3 are produced in turn by the action of appropriate synthases. Subsequently, GM3, GD3 and GT3 serve as precursors of more complex gangliosides by the action of further glycosyl- and sialyl-transferases. An alternative theory with some supporting evidence proposes that a multiglycosyl-transferase complex is responsible for the synthesis of each individual ganglioside rather than a series of individual enzymes. Further sialylation of each of the a, b, and c series and in different positions in the carbohydrate chain can occur to give an increasingly complex and heterogeneous range of products, such as the α-series gangliosides with sialic acid residue(s) linked to the inner N-acetylgalactosamine residue (not illustrated). GM4 or NeuAcα2,3Gal-Ceramide, a minor component of the brain and present in a few other tissues at low levels, is an exception in that galactosylceramide is its precursor. Finally, the newly synthesized gangliosides are transferred to the external leaflet of the plasma membrane via the lumenal surface of transport vesicles. Gangliosides are also important constituents of nuclear membranes.
The changes that occur in ganglioside compositions of brain and other tissues in the embryonic and post-natal stages are governed mainly by changes in the expression level and activity of the glycosyl- and sialyl-transferases, although the former can also be regulated by glycosylation and phosphorylation.
The presence of distinctive sialidases that differ from the catabolic lysosomal enzymes (see below) in raft-like regions of the plasma membrane bring about further changes in the composition of the cell surface gangliosides that can be specific to particular cell types, causing a shift from poly-sialylated species involving a decrease of GM3 and formation of GM2 then GM1 by hydrolysis of terminal sialosyl residues linked either α2‑8 on another sialic acid or α2‑3 on galactose. As GM1 is resistant to most sialidases, it tends to increase in concentration relative to oligosialo species as developmental and other GM1-requiring processes come into play. This may have consequences for important cellular events, such as neuronal differentiation and apoptosis. Conversely, sialylation may occur in some neuronal membranes, increasing the proportions of poly-sialylated species. In particular, a CMP-NeuAc:GM3 sialyltransferase is able to sialylate GM3. Gangliosides GM1 and GD1a have been identified in both membranes of the nuclear envelope together with two neuraminidases.
Ganglioside lactones, where the sialic acids are linked together with ester linkages, have been detected as minor components in brain tissues, where lactonization occurs at the plasma membrane. As the process of lactonization profoundly influences the shape and biological properties of the original ganglioside, it is possible that lactonization-delactonization in a membrane might be a trigger for specific cellular reactions. Similarly, GD3 ganglioside can undergo O-acetylation at C9 of the outer sialic acid with important metabolic implications.
Gangliosides added to many types of cell preparations in vitro are rapidly taken up by the cells, while gangliosides injected into animals in vivo are rapidly internalized by tissues. They can cross the blood-brain barrier, and via the placenta, they can enter the fetus. Similarly, dietary gangliosides are absorbed intact by intestinal cells but are broken down to their lipid and carbohydrate constituents for re-use. The sialic acids released by an intestinal sialidase are transported in plasma to the brain and other tissues where they influence ganglioside expression. Indeed, there is some experimental evidence that dietary gangliosides may improve cognitive functions in animals and humans.
Catabolism
Degradation of gangliosides takes place at the surface of intralysosomal luminal vesicles, generated by an inward budding of the endosomal membrane, and these are reached by a process of endocytosis. In brief in relation to gangliosides, soluble sialidases (neuraminidases) and exoglycohydrolases remove individual sialic acid and sugar residues sequentially from the non-reducing terminal unit, as illustrated for ganglioside GM1, with the eventual formation of ceramide, which is then split into long-chain base and fatty acids by ceramidases. This degradation occurs through the endocytosis-endosome-lysosome pathway with a requirement for an acidic pH inside the organelle. In addition to the sialidases and exoglycohydrolases, the various reactions have an absolute requirement for effector molecules, termed 'sphingolipid activator proteins', including saposins (Sap), and the specific GM2-activator protein (GM2-AP). Ganglioside GM3 is a component of the lysosomal perimeter membrane, but is protected from degradation by a glycocalix of the membrane facing the lysosol. Anionic lipids and especially bis(monoacylglycero)phosphate in the membranes stimulate ganglioside degradation while cholesterol is inhibitory. The catabolic pathway is shown in Figure \(\PageIndex{30}\).
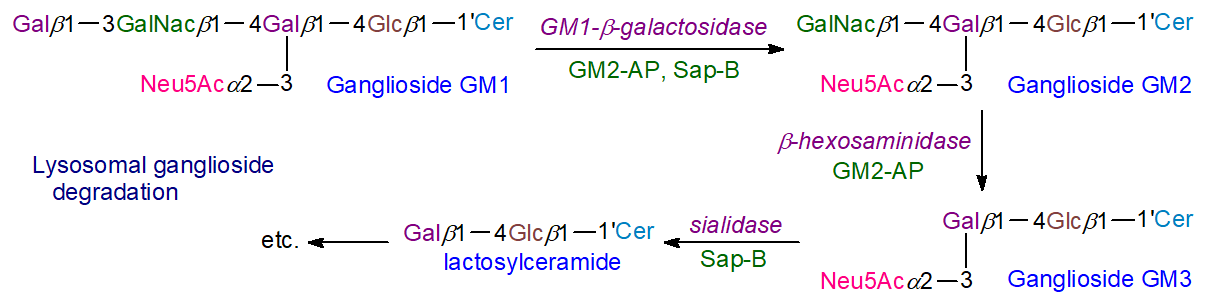
This process constitutes a salvage mechanism that is important to the overall cellular economy since a high proportion of the various hydrolysis products are recycled for glycolipid biosynthesis. By generating ceramide and sphingosine, it may also be relevant to the regulatory and signaling functions of these lipids. In addition, some partial hydrolysis of gangliosides occurs in the plasma membrane as part of a biosynthetic remodeling process discussed above. Defects in catabolism lead to the gangliosidoses discussed later.
Ganglioside Function
Cell surface effects: In their natural biological environment, gangliosides have a negative charge because of the presence of sialic acids, which also add to the hydrophilicity of the polysaccharide constituent. This is balanced somewhat by the hydrophobic character of the ceramide moiety, so that over all the molecules are amphiphilic in nature, but very different from the glycerophospholipids, which are essential for the formation of membrane bilayers. Indeed, a ganglioside such as GM1 is virtually soluble in water, where it can form large aggregates though hydrophilic effects. The nature of the ceramide unit with its capacity to form hydrogen bonds with glycerophospholipids is important in ensuring that gangliosides are inserted in a stable manner into the outer layer of the plasma membrane.
Thus, gangliosides are anchored in membranes by their ceramide units with the double-tailed sialoglycan components extending out from the cell surface, where they can participate in intermolecular interactions by a network of hydrogen bonds and hydrophobic interactions. For example, the glucose-ceramide bond of GM1 is oriented in the outer leaflet of the plasma membrane such that the glycan extends perpendicularly to the plane of the lipid bilayer. All gangliosides, but especially the simplest GM3 or Neu5Acα2-3Galβ1-4Glcβ1Cer, have a structural role, and they a natural propensity to laterally segregate and to associate with each other and with other sphingolipids, phospholipids and cholesterol into raft nano-domains or in related structures, such as the caveolae, where the very large surface area occupied by the oligosaccharide chain imparts a strong positive curvature to the membrane. In this environment, gangliosides can interact with each other through side-by-side hydrogen bonds mediated by water molecules that act as bridges between the chains.
Further, molecules of GM3 and other gangliosides self-aggregate into clusters on the surface of lymphocytes of human peripheral blood, and there is evidence that the density of these clusters in membranes governs their reactivity as antigens. In addition, it is believed that gangliosides and other oligoglycosylceramides can cluster together through hydrogen donor-acceptor (cis) interactions because of the presence of hydroxyl and acetamide groups to form glycosynaptic domains, which are related to but functionally distinct from raft signaling platforms (with lower cholesterol concentrations). Many of the biological functions of gangliosides are mediated through their location in these nanodomains, where they may have specialized functions in cell adhesion, growth, and motility through interactions with specific proteins and signal transduction pathways. However, not all gangliosides are present in such raft-like structures.
Receptor/signaling functions: Gangliosides can bind to membrane proteins directly by carbohydrate-carbohydrate or carbohydrate-amino acid interactions, usually involving specific ganglioside head groups, resulting in changes to the location of proteins within membrane microdomains for recruitment of signaling partners, or to dimerization or other effects upon receptors. In rafts and caveolae especially, gangliosides can modulate cell signaling processes by their interactions with specific receptors, adhesion molecules, and ion channels. Cell–cell (trans) interactions occur by sialoglycans on one cell binding to complementary binding proteins (lectins) on adjacent cells, bringing about adhesion of cells and enabling regulation of intracellular signaling pathways, e.g. myelin-associated glycoprotein on myelin sheaths binds to gangliosides present on axonal membranes.
In addition, gangliosides act as receptors of interferon, epidermal growth factor, nerve growth facto,r and insulin, and they may regulate cell signaling and control growth and differentiation of cells in this way. While intact gangliosides inhibit growth by rendering cells less sensitive to stimulation by epidermal growth factor, removal of the N-acetyl group of sialic acid enhances this reaction and stimulates growth. Gangliosides function as antigens or receptors by recognizing specific molecules (lectins), including bacterial toxins, at the cell surface and by modulating the charge density at the membrane surface (see the section on Gangliosides and Disease below). They also regulate the activities of proteins within the plasma membrane and especially receptor-type tyrosine kinases. For example, the phosphorylation state and activity of insulin receptors in caveolae and thence the insulin resistance of cells is controlled by the concentration of GM3, the main ganglioside in plasma and other extraneural tissues. GM3 interacts also with the epidermal growth factor receptor leading to cell growth inhibition. GM1 strongly influences specific neuronal functions by interacting with specific receptors such as the tropomyosin receptor kinase (Trk) A (TrkA) receptor by altering its conformation to enable interaction with the nerve growth factor (NGF) ligand.
GM3 (SA-Gal-Glc-Cer) is a serum ganglioside that is highly enriched in a type of membrane microdomain termed a 'glycosynapse', and it forms complexes with co-localized cell signaling molecules. It has a function in the innate immune function of macrophages and it has been demonstrated that molecular species of GM3 with differing acyl-chain structures and modifications can operate as pro- and anti-inflammatory modulators of Toll-like receptor 4 (TLR4); very-long-chain and α-hydroxy GM3 species increase TLR4 activation, while long-chain and unsaturated GM3 species have the opposite effect. In addition, gangliosides have been shown to be cell-type specific antigens that have key functions in immune defense. For example, a major immunological function of gangliosides and sialic acids is to protect cells from attack by our own immune system and from autoimmunity. They recognize and protect host organs and tissues from complement attack by binding to the complement regulatory protein factor H, which has the potential to exert strong cytotoxic and inflammation-inducing activity. In particular, sialic acids protect against complement killing of autologous cells by binding to this protein via the α2–3 linked sialic acid glycans of the GD3 ganglioside. On the other hand, the breakdown of this system can lead to autoimmune diseases.
Brain function: One of the first examples of a ganglioside influencing a signaling event to be studied in some detail concerns the simple ganglioside GD3, which has a central role in early neurogenesis. GD3 binds to the epidermal growth factor receptor (EGFR) via a protein-carbohydrate interaction involving its terminal N-acetylneuraminic acid and a lysine residue in the transmembrane domain of the receptor and also by a carbohydrate-carbohydrate interaction thereby maintaining the latter in its inactive monomeric state. EGFR then binds to the epidermal growth factor and stimulates the transition of the receptor from an inactive monomeric to an active homodimeric form, and this in turn triggers receptor auto-phosphorylation and activation of a signaling cascade that promotes cell proliferation. This has proven to be essential for the regulation of the stem cell self-renewal capacity in the brain. In contrast, the neutral oligoglycosphingolipid Gb4 exerts the opposite effect on EGFR by interacting directly with it to potentiate its auto-phosphorylation with activation of the downstream cascade.
The techniques of molecular biology such as targeted gene deletion, which enable specific enzymes to be eliminated from experimental animals, are now leading to a better understanding of the function of each ganglioside. It is evident that they are essential to central myelination, to maintain the integrity of axons and myelin, and for the transmission of nervous impulses. These effects may be mediated by interactions of the negatively charged sialic acid residues of gangliosides with calcium ions, which are critical for neuronal responses. For example, a variant of GD3, 9-O-acetyl GD3, appears to be involved in glial-guided neuronal migration during brain development in the rat, while GM1 may have a similar function in humans; it determines which growth cone of unpolarized neurons becomes the axon. By stabilizing neuronal circuits, gangliosides have a function in memory, and conversely, disturbances in ganglioside synthesis can lead to neurodegenerative disorders (see below). Ganglioside GM3 in raft domains has been shown to have an indispensable role for the development, function, and viability of cochlear hair cells and thence it is essential for hearing. On the other hand, mice that express GM3 primarily and are devoid of the typical complex gangliosides of the brain suffer weight loss, progressive motor and sensory dysfunction, and deterioration in spatial learning and memory with aging. GD3 is important for retinal structure and visual function in mice.
Changes in ganglioside composition can be induced by nerve stimulation, environmental factors, or drug treatments. The various interconvertible ganglioside types in the plasma membrane of neurons are particularly important for its development in that they regulate such processes as axonal determination and growth, signaling, and repair. In addition, gangliosides are believed to be functional ligands for the maintenance of myelin stability and the control of nerve regeneration by binding to a specific myelin-associated glycoprotein. The occurrence of gangliosides in cell nuclei suggests a possible involvement of gangliosides in the expression of genes relevant to neuronal function. For example, the monosialoganglioside GM1 has been shown to promote the differentiation of various neuronal cell lines in culture. It has protective effects on the neural system by encouraging neural stem cell survival and proliferation, while facilitating the stability and regeneration of axons, and by inhibiting neurodegeneration through autophagy, for example after ischemic stroke. Within membrane rafts, this ganglioside has key roles in several signaling systems through association with specific proteins that have glycolipid-binding domains, including those that modulate mechanisms such as ion transport, neuronal differentiation, G protein-coupled receptors (GPCRs), immune system reactivities and neuroprotection. It is important for Ca2+ and Na+ homeostasis in the nucleus and plasma membrane and in regulating the effects of platelet-derived growth factor. However, there have been unpleasant complications when GM1 has been administered for therapeutic purposes. GD1a is sometimes considered to be a reserve pool for GM1.
After nerve injury, toll-like receptor 2 (TLR2) signaling is important for the induction of neuropathic pain; ganglioside GT1b functions as a TLR2 agonist to produce mechanical and thermal hypersensitivity.
Other functions: The ganglioside GD3 is essential for the process of apoptosis by blocking the activation of specific transcription factors and thence disabling the induction of antiapoptotic genes. 9-O-Acetylation of the GD3 molecule prevents ganglioside oxidation and blocks its pro-apoptotic effects. Similarly, GD3 is a regulator of autophagy, i.e. the degradation and/or recycling of cellular components. Gangliosides are also important in reproduction, and in mice, GD1a has been shown to be important to oocyte maturation, monospermic fertilization, and embryonic development, while GM1 is important in sperm-oocyte interactions and sperm maturation processes. Deletion of the GM2/GD2 synthase leads to infertility in male mice and the production of a novel fucosylated ganglioside containing very-long-chain polyunsaturated fatty acids. Related studies with gene knockout mice have revealed that b-series gangliosides are important in leptin secretion from adipocytes, while a-series gangliosides interact with the leptin receptor in the hypothalamus to influence the balance of energy.
Gangliosides and Disease
Bacterial toxins and viruses: In relation to adaptive immunity, a-series and o-series gangliosides in the plasma membrane are involved in the function and stimulation of receptors on certain subsets of T cells by acting as pattern-recognition receptors for invading pathogens. In particular, certain gangliosides bind specifically to viruses and to various bacterial toxins, such as those from botulinum, tetanus and cholera, and to blood merozoites of the deadliest malaria parasite Plasmodium falciparum, and they mediate interactions between microbes and host cells during infections, with NeuAc as the main recognition module. The best known example is cholera toxin, which is an enterotoxin produced by Vibrio cholerae where the specific cell surface receptor is ganglioside GM1; the five B-chains of cholera toxin each bind one molecule of GM1. Interestingly, the subsequent metabolism of the ganglioside-toxin complex is dependent on the nature of the fatty acid components of the ganglioside. It is believed that toxins utilize the gangliosides to hijack an existing retrograde transport pathway from the plasma membrane to the endoplasmic reticulum. For example, the passage of the cholera toxin through the epithelial barrier of the intestine is mediated by GM1, possibly by endocytosis of the toxin-GM1 complex via caveolae into the apical endosome and thence into the Golgi/endoplasmic reticulum, where the complex dissociates. The consequence is persistent activation of adenylate cyclase by the toxin and continuous production of cAMP that leads to the severe fluid loss typical of cholera infections. As a further example, the botulinus toxin binds to a complex of a polysialoganglioside with the protein synaptotagmin, which together act as a high-affinity receptor complex to enable the neurotoxic effects. Similarly, ganglioside GM2 binds to a toxin secreted by Clostridium perfringens.
Influenza viruses have two glycoproteins in their envelope membranes, hemagglutinins, which bind to cellular receptors such as gangliosides, and after entry into respiratory epithelial cells, the sialidase (neuraminidase) of the virus cleaves the sialic acid from the receptors to prevent entry of further viruses to the cell. Variations in the structure of these proteins force the development of new vaccines The carbohydrate moiety of gangliosides is essential for the initial binding of viruses, but the lipid moiety is believed to be important for controlling their intracellular transport.
Some gangliosides and GD1a especially have anti-inflammatory properties in that they inhibit the effects of bacterial lipopolysaccharides by preventing the activation of tumor necrosis factor (TNF) and other cytokines. In contrast, GM2 may increase cytokine production in similar circumstances, while the heat-labile toxins of Escherichia coli bind to several gangliosides in macrophages, thus activating an inflammatory response.
Gangliosidoses and other neurodegenerative diseases: It appears to be a general rule that the mere process of lysosomal substrate accumulation in all lysosomal storage disorders impairs lysosome integrity and results in more general disruptions to lipid metabolism and membrane structure and function, inevitably triggering pathologic mechanisms. Endogenous generation of antibodies to gangliosides is often a factor, and it has been argued that gangliosides and their sialic acids components are at the border of immune tolerance.
As with the neutral oligoglycosylceramides and ceramide monohexosides, a number of unpleasant lipidoses have been identified that involve the storage of excessive amounts of gangliosides in tissues because of failures in the catabolic mechanism. The most important of these are the GM2 gangliosidoses, i.e. Tay-Sachs disease (and the similar Sandhoff disease), a fatal genetic disorder found mainly in Jewish populations in which harmful quantities of ganglioside GM2 accumulate in the nerve cells in the brain and other tissues. Lyso-GM2 (non-acylated) in plasma may serve as a marker. A modified GM2 derivative that contains taurine in amide linkage to the sialic acid carboxyl group has been identified in the brain of such patients. As infants with the most common form of the disease develop, the nerve cells become distended and a relentless deterioration of mental and physical abilities occurs. The condition is caused by insufficient activity of specific enzymes, i.e. β‑N‑acetylhexosaminidase, which catalyzes the degradation of gangliosides by removing the terminal N-acetylgalactosamine residue from GM2, or the GM2 activator protein.
In addition, a generalized GM1 gangliosidosis (an autosomal recessive and neurodegenerative disease) has been characterized in which ganglioside GM1 accumulates in the nervous system leading to mental retardation and enlargement of the liver. The condition is a consequence of a deficiency of the lysosomal β-galactosidase enzyme, which hydrolyses the terminal β-galactosyl residues from GM1 ganglioside to produce GM2. It appears that storage of substantial amounts of unwanted lipids in the lysosomal system leads to a state of cellular starvation, so that essential elements such as iron are depleted in brain tissue. The presence of lyso-GM1 in plasma is now seen as a useful aid to diagnosis. Small amounts of some gangliosides accumulate as secondary storage compounds in Niemann–Pick disease. The Guillain–Barré syndrome is an acute inflammatory disorder, usually triggered by a severe infection, which affects the peripheral nervous system. Antibodies to gangliosides are produced by the immune system, leading to damage of the axons, which can result in paralysis of the patient. Huntington’s disease is believed to involve disruption of the metabolic pathways between glycosylceramides and gangliosides, and there is a human autosomal recessive infantile-onset epilepsy syndrome caused by a mutation to a sialyl transferase. Impaired ganglioside metabolism is also relevant to Alzheimer’s disease, because complexation with ganglioside GM1 may cause aggregation of the amyloid β-protein deposits that characteristically accumulate in the brain in this condition (this explanation does not appear to be universally accepted). In general, in ganglioside deficiencies, natural or induced, it appears that progressive inflammatory reactions take place, leading to neurodegeneration in part because of the deterioration of the architecture of lipid rafts.
On the other hand at normal tissue concentrations, gangliosides such as GM1 are believed to have an anti-inflammatory and neuroprotective role in certain types of neuronal injury, Parkinsonism, and some related diseases. For example in relation to Parkinson's disease, GM1 binds to α-synuclein and inhibits or eliminates fibril formation. It may have a protective role by preventing sphingomyelin-induced aggregation, although as the overall level of GM1 decreases during aging, its beneficial effect decreases. For these reasons, the therapeutic properties of ganglioside GM1, the most accessible species, and derived molecules are under clinical investigation. However, there is no approved therapy for any gangliosidosis, although a number of different therapeutic strategies are being studied, including hematopoietic stem cell transplantation and gene therapy. For the moment, the blood-brain barrier remains a challenge.
Cancer: Gangliosides have important functions in cancer, especially in the regulation of signal transduction induced by growth-factor receptors in a specific microdomain termed a 'glycosynapse' in the cancer cell membranes, and in interactions with glycan recognition molecules involved in cell adhesion and immune regulation. In particular, depending on tissue, certain distinctive gangliosides are expressed at much higher levels in tumors than in normal healthy tissues, mainly by aberrant expression of glycosyltransferases and glycohydrolases. This enables tumor cells to escape immune surveillance and retain their malignancy. GM3 is not expressed in melanocytes normally, but is detected in 60% of primary melanomas and in 75% of metastatic melanomas, for example. Gangliosides can be shed from the surface of tumor cells into the local environment where they can influence interactions between cancer cells, including the transition of tumors from a dormant to a malignant state (angiogenesis); when present in the circulation they can be useful diagnostic aids. For example, the ganglioside GM3 is elevated in the serum of patients with breast cancer and may be a biomarker for the disease, while disialylated gangliosides GD2 and GD3 (Figure \(\PageIndex{31}\)) are considered to be markers of neuroectoderm origin in tumors (neuroblastoma).
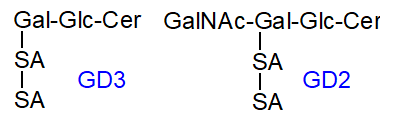
Specific gangliosides can have either positive or negative effects upon the regulation of the malignant properties of cancer cells. As a generality, disialyl glycosphingolipids or tandem-repeated sialic acid-structures confer malignant properties in various cancer systems; they are not merely markers. For example, the disialo-gangliosides GD2 and GD3 are present in trace amounts only in normal tissues, but are found at much higher concentrations in cancer cells, especially melanomas and neuroblastomas, with GD2 especially elevated in triple-negative breast cancer. These b-series gangliosides play a substantial part in the malignant properties of gliomas by mediating cell proliferation, migration, invasion, adhesion, and angiogenesis, and in preventing immunosuppression. They are considered to be tumor-associated antigens, and the GD2 and GD3 synthases are seen as important drug targets. In contrast, monosialyl gangliosides, such as GM1, GM2 and GM3, may suppress the malignant properties of various cancer cells. The mechanism is believed to involve complex formation at the cell surface with membrane proteins, such as growth factor receptors and adhesion receptors like those of the integrin family, leading to the modification of cell signals mediated by these receptors. Metastatic melanoma cells have high levels of GD3 in comparison to poorly metastatic cells or the normal counterpart, suggesting that GD3 may promote metastasis possibly by suppressing the anti-tumor immune response.
Ganglioside GM3(Neu5Gc), i.e. containing an abnormal sialic acid, is sometimes considered to be a tumor-specific antigen and a target for cancer immunotherapy. Aberrant sialylation is found in many malignant cancers, where the levels of neuraminidases are key factors for metastasis and survival of cancer cells, and there can be a significant accumulation of unusual gangliosides containing N-glycolyl sialic acid in some cancers. N-Glycolyl-GM3, normally absent from human tissues, is present in all stage II breast cancers, and it is accompanied by a number of other less common complex gangliosides. Similarly, the 5-N-deacetylated form of GM3 is expressed in metastatic melanomas, but not in healthy tissue or even in primary melanomas; it is considered to be a specific marker for the metastatic condition and a target for potential therapy. Increased synthesis of 9-O-acetyl-GD3, dependent on a sialyl-O-acetyltransferase - CAS1 Domain-Containing Protein 1, occurs in acute lymphoblastic leukemia and in malignant melanomas, and this appears to limit apoptosis, while O-acetylated GD2 (OAcGD2) is expressed in breast cancer and other tumors. A unique fucosyl-GM1 in which the terminal galactose is α-1,2-fucosylated at the non-reducing end is found circulating in the serum of patients with a number of cancers and especially with small-cell lung cancer but rarely in normal conditions, and it is also considered to be a potential indicator of cancer and a candidate for immunotherapy.
Clinical trials with an antibody to GD2 have been carried out successfully against the rare childhood cancer neuroblastoma, and the USDA has approved the use of this in combination with other drugs to treat this often lethal cancer. However, this antibody can have painful side effects due to an interaction with GD2 on neurons, and modified antibodies, which may be safer, are now being tested in multiple clinical trials. A phase I clinical trial with an antibody to GD3 has shown promising results in patients with malignant melanoma. Similarly, antibodies to OAcGD2 and fucosyl-GM1 have shown anti-tumour effects in vitro, and studies with human patients are underway.
Other diseases: Aberrant production of the ganglioside GM3 has been linked to pathophysiological changes associated with obesity, metabolic disorders, and type 2 diabetes mellitus through its effects on insulin receptors. It has a role in autoimmune disorders such as multiple sclerosis. In epilepsy, it is believed that a deficiency in the enzyme ceramide synthase 1, which produces 18:0 ceramides, leads to reduced ganglioside formation. By their presence in certain subsets of T cells, gangliosides influence allergic responses and auto-immune diseases. As gangliosides are present on the surface of vascular, vascular-associated, and inflammatory cells, they may have a role in atherosclerosis and in aging.




.png?revision=1&size=bestfit&width=417&height=294)
.png?revision=1&size=bestfit&width=297&height=378)