28.8: Receptor Guanylyl Cyclases, cGMP, and Protein Kinase G
- Page ID
- 76554
Search Fundamentals of Biochemistry
Much of this material is derived from Friebe et al. cGMP: a unique 2nd messenger molecule – recent developments in cGMP research and development. Naunyn-Schmiedeberg's Archives of Pharmacology volume 393, pages 287–302 (2020). Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/
Introduction
We have considered many signal transduction pathways, starting with an extracellular signal, a primary messenger, that initiates signaling when it binds to a receptor (GPCR, RTK, Cytokine Receptor, etc). It elicits a conformation change in the receptor, which is transmitted to an intracellular domain, from where it can propagate and transmits a signal intracellularly through secondary messengers and phosphorylation of signaling proteins. Some primary messengers, however, actually pass through the cell either passively or through membrane carriers, so there is no need to generate a second messenger. We will consider two signals that translocate through the cell membrane, gases such as nitric oxide (NO), and steroid hormones (which we will discuss in a future section). NO produced within a cell activates the formation of an intracellular second messenger, cyclic GMP (analogous to cAMP). cGMP in turn activates our final member of the AGC Ser/Thr protein kinase family, protein kinase G (PKG). Intracellular NO has an unexpected role on adjacent cells. Given its small size and its nonpolar nature, it can diffuse out of the cell where it is produced and into an adjacent cell, where it can also initiate signaling. Some call this retrograde signaling.
Before we consider cGMP and PKG, let's see how nitric oxide (NO) is produced.
NO formation
NO is synthesized by the enzyme nitric oxide synthase (NOS). There are three isoforms in mammals, neuronal (NOS1 or nNOS), inducible (NOS2 or iNOS), and endothelial (NOS3 or eNO2). Each is a homodimer with a complex domain structure, including a
- N-terminal oxidase (NO_synthase) domain that binds heme
- calmodulin binding site between the N and C terminal domains
- C-terminal reductase domain containing the FMN (Flavodoxin_1) subdomain (which contains an autoinhibitory helix) and FAD/NADPH subdomain.
Ca2+ ion activates the enzyme through the binding of Ca2+/CAM. A more detailed description of the domain structures of human NOS is shown in Figure \(\PageIndex{1}\). The dimeric molecular weight of the neuronal NOS1 (321K) is greater than for iNOS (206) and eNOS (266) as it has a N-terminal PDZ domain.
| neuronal NOS 1 (MW 321) |  purple NADPH binding purple NADPH binding |
|
inducible (MW 206K) endothelial (MW 266) |
 yellow NADPH binding yellow NADPH binding |
Figure \(\PageIndex{1}\): Domain structure of human nitric oxide synthases
NOS catalyzes the conversion of the free amino acid arginine to citrulline and NO, as shown in the chemical equation in Figure \(\PageIndex{2}\).
The structures of the three enzymes with or without bound CAM are similar. Linkers between the domains and subdomains allow flexibility. Figure \(\PageIndex{3}\) shows the flow of electrons from NADPH into the reductase (NADPH/FAD subdomain to the FMN subdomain), and on to the NOS synthase domain containing the heme.
Two monooxygenase reactions occur in NOS synthase (oxidative domain) in which electrons are funneled into the heme and bound dioxygen (O2) leading to the formation of water and the final product, NO. Electron transfer only occurs within the dimer when calmodulin is bound. However, iNOS is active even at basal Ca2+ concentrations.
Figure \(\PageIndex{3}\) shows an interactive iCn3D model of the structure of human neuronal nitric oxide synthase (with its PDZ domain) predicted by AlphaFold (P29475).
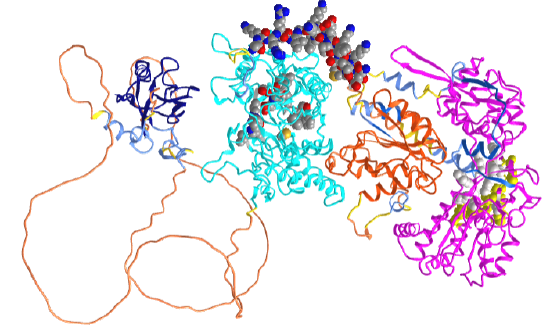
In the iCn3D model, orient the protein as shown in the figure above. The dark blue (left) is the PDZ domain, and cyan is the oxidase (NOS synthase) domain that contains the heme (which is not shown in AlphaFold models). The spacefill CPK color shown in the cyan domain is active site residues interacting with the heme (not shown). The orange domain is the FMN (Flavodoxin_1) subdomain in the reductase domain. The magenta (far right) shows the FAD/NADPH subdomains of the reductase domain. The yellow spacefill shows the NAD binding pocket and the white spacefill the FAD binding pocket. The structures of amino acids 129-304 between the PDZ domain and the oxidase domain are not predicted with any certainty. Crystal structures are available for the oxidase domain alone. The spacefill CPK-colored helix (amino acid 730-754) represents a helical peptide region that binds to calmodulin.
NO that is synthesized in the cells can signal there or diffuse to another cell and signal there. Figure \(\PageIndex{11}\) shows how NO synthesized in vascular epithelial cells that line blood vessels can move into the nearby muscle cells and initiate signaling through soluble guanylyl cyclase there, leading to vasodilation and a lowering of blood pressure.
Endothelial nitric oxide synthase (eNOS) located in the vascular endothelium forms NO from plasma arginine. Two substrates, O2 and NADPH, are required along with the cofactors tetrahydrobiopterin (BH4), FAD, and flavin mononucleotide (FMN). NO diffuses into smooth muscle cells and activates soluble guanylyl cyclase (sGC), increasing cGMP production. cGMP subsequently activates protein kinase G (PKG), resulting in decreased [Ca2+] by these mechanisms:
- inhibition of voltage-dependent calcium channels (VDCC), reducing calcium influx;
- activation of plasma membrane calcium ATPases (PMCA), increasing ATP-dependent calcium efflux;
- inhibition of inositol triphosphate receptors (IP3R), reducing calcium release from the sarcoplasmic reticulum (SR) to the cytoplasm;
- activation of sarcoplasmic calcium ATPases (SERCA), increasing the ATP-dependent sequestration of calcium from the cytoplasm to the SR.
Decreased [Ca2+] mediates smooth muscle relaxation via the activation of myosin light chain kinase and the inhibition of myosin light chain phosphatase (not shown in the figure), resulting in vasodilation.
Figure \(\PageIndex{13}\) shows retrograde diffusion of NO from an activated post-synaptic neuron back to the presynaptic neuron that excited it on the release of the neurotransmitter glutamate. NO is synthesized by post-synaptic cell nNOS after it's activated by Ca2+ inflow and binding to CAM (not shown). NO with the post-synaptic neuron binds to guanylyl cyclase to produce the second messenger cGMP which can directly activate other channels, protein kinase G, or phosphodiesterases (PDEs)
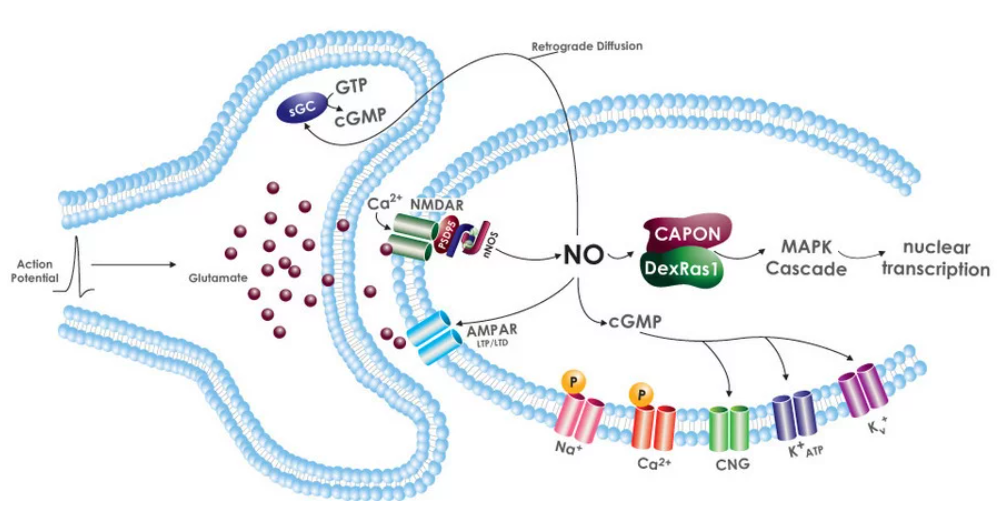
Synaptic glutamate release activates postsynaptic NMDA and AMPA receptors (NMDAR, AMPAR) leading to Ca2+-induced nNOS activation. NO will diffuse back to the presynaptic cell and activate sGC to produce cGMP, which has many signaling roles including affecting presynaptic neurotransmitter release. cGMP directly targets several ion channels in the post-synaptic cell. As we saw in the previous chapter, many ion channels are voltage-gated. However, ion channels can be regulated directly by ions (ex Ca2+, Na+) as well as by cyclic nucleotides such as cAMP and cGMP. The later channels are called cyclic nucleotide-gated (CNG) channels. NO in the post-synaptic cell also associates with CAPON, a nNOS binding protein, leading to downstream MAP kinase cascade.
Everyone knows that carbon monoxide (CO) in high doses is lethal as it binds to heme Fe2+ in hemoglobin and myoglobin with a higher affinity than O2. Hence it may come as a surprise to you that endogenous CO is a signaling molecule, which now in retrospect might make sense given its similarity in chemical structure to NO.
CO is produced through heme oxygenase (HOs). CO can act as a signaling molecule in neural, cardiovascular, respiratory, gastrointestinal, immune, and reproductive systems. In contrast to the lethal effects of inhaling exogenous CO from incomplete combustion, endogenous CO has anti-inflammatory and antioxidant effects. It can also act to dilate the vasculature system. Other gases like H2S also are signaling agents.
cGMP formation
The second (or third) messenger cyclic guanosine monophosphate (cGMP) is synthesized after activation of the enzyme guanylyl cyclase (GC) by nitric oxide. cGMP has many signaling effects in cells, some of which were outlined above. The cytoplasmic soluble GC (sGC) is activated by NO. The membrane-associated "particulate" GC (pGC) form is activated on the binding of natriuretic peptides (NPs) to natriuretic peptide receptors, which are NP-activated integral membrane guanylyl cyclase. The peptide hormones (ANP secreted by the atria and BNP secreted by ventricles) decrease blood pressure. The membrane form does not require NO for activation Figure \(\PageIndex{14}\) shows the conversion of GTP to cGMP.
Synthesis of cGMP from soluble GC is activated by NO or molecules like nitrates that can be metabolized to NO. These molecules are called NO donors. Since NO causes vasodilation, they are used to treat angina and hypertension. A class of drugs called stimulators (for example riociguat), increases cGMP production from sGC in the absence and synergistically in the presence of NO. They are also used to treat hypertension. Another class of drugs called activators can activate sGC even if heme is oxidized or even missing without upstream NO signaling. They are effective even if the heme is oxidized or lost from the NOS catalytic domain.
We saw that cAMP is cleaved to AMP by phosphodiesterase. Likewise, phosphodiesterases (PDEs) cleave cGMP to GMP to attenuate signaling through cGMP. Selective drugs targeting PDE are available. These include sildenafil for the treatment of pulmonary hypertension and erectile dysfunction and tadalafil for benign prostatic hyperplasia (BPH).
Pathways for activation of guanylyl cyclase activity (sGC and pGC) are shown in Figure \(\PageIndex{15}\).
Soluble guanylyl cyclase (cGC) structure and function
The soluble form of GC is a heterodimer of α and β subunits. The domain structure of guanylyl cyclase is shown in Figure \(\PageIndex{16}\).
It appears that when NO binds to the heme group, a twist in the coiled-coil domain leads to its extension, which leads to the activation of the catalytic domain. Simulators likely cause similar conformational changes initiated by their binding to the top part of the CC domain. Figure \(\PageIndex{17}\) shows an interactive iCn3D model of the human soluble guanylate cyclase in the riociguat (stimulator) and NO-bound state (7D9R)
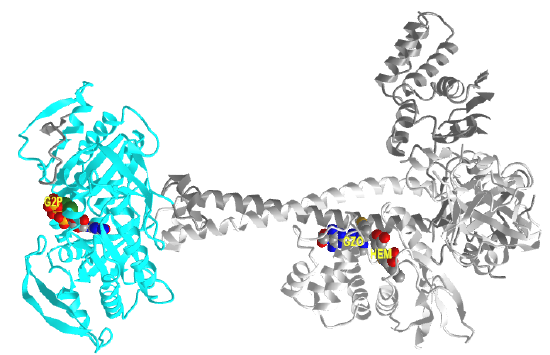
The A chain is dark gray and the B chain is light gray. The guanylate cyclase domain contributed to by each monomer is shown in cyan. A phosphonate GTP analog (labeled G2P) is shown in spacefill CPK color bound in the guanylyl cylase domain (cyan). The heme (HEM) and the stimulator riociguat (GZO) are shown in the HNOBA (Heme NO Binding Associated) domain and shown in spacefill CPK colors.
The conformation change of A chain of the bent inactive form of guanylyl cyclase (6JT1) to the active extended form (6JT2) is shown in Figure \(\PageIndex{18}\).
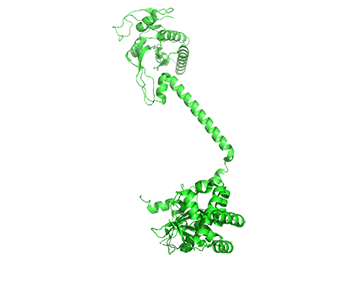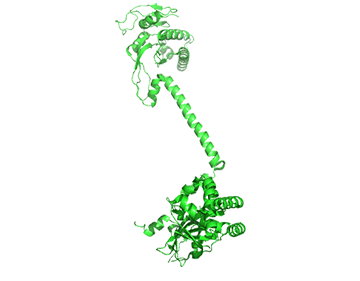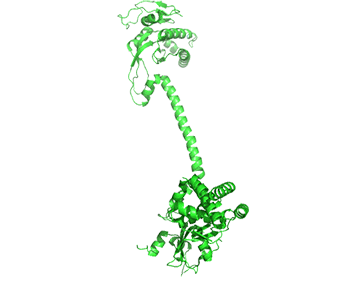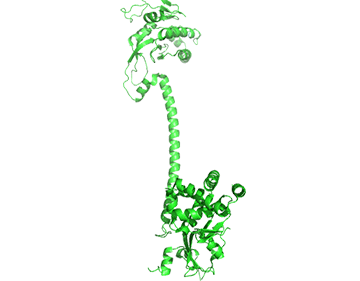
The guanylyl cyclase domain is at the top of the figure. The conformational change is somewhat reminiscent of the change in apo-calmodulin on the binding of Ca2+ ions, although the central helix is fully intact in the inactive and active forms of guanylyl cyclase.
Figure \(\PageIndex{19}\) shows how oxidation of the heme iron and S-nitrosation of the protein that occurs in the presence of reactive oxygen species (ROS) and reactive nitrogen species (RNS) leads to heme loss and inactivation of sGC.
pGC structure and function
The other source of cytosolic cGMP is particulate guanylyl cyclase (pGC). These are integral membrane protein receptors for natriuretic peptides (NPs), which when bound to the receptor activate the cytoplasmic guanylyl cyclase domain of the receptor. In effect, they are ligand (NPs)-gated receptor enzymes. The peptide hormones (ANP secreted by the atria and BNP secreted by ventricles) decrease blood pressure. There are seven variants of pGC (A-G) found in mammals. GC-A (also called NPR-A or NPR1) and GC-B (NPR-B or NPR2), are both receptors for natriuretic peptides. The domain structure of NPR-A is shown in Figure \(\PageIndex{20}\).

Figure \(\PageIndex{21}\) shows an interactive iCn3D model of the human atrial natriuretic peptide receptor1 AlphaFold predicted model (P16066)
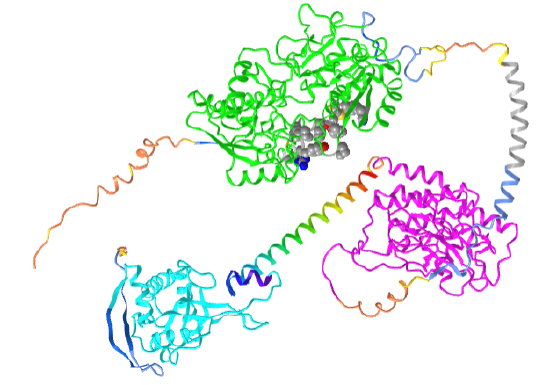
Orient the model as shown in the figure above. The coloring coding in the model is as follows:
- green: Ligand (ANP)-binding domain of the type A natriuretic peptide receptor (NPR-A);
- magenta: PK-GC pseudokinase domain;
- cyan: cyclase domain;
- rainbow helix: HNOBA domain is found to be associated with the HNOB domain and pfam00211 in soluble cyclases and signaling proteins. The HNOB domain is predicted to function as a heme-dependent sensor for gaseous ligands rainbow;
- gray helix: transmembrane segment amino acids 474-494.
Note the protein is an integral membrane protein that passes through the membrane using a single alpha helix (474-494). The N-terminal domains above it and the C-terminal domains below it would orient like a typical single-pass membrane protein in the presence of a bilayer.
Protein Kinase G (PKG)
To briefly review, NO production leads to the production of cGMP. cGMP can directly bind to and regulate membrane ion channels. In alignment with the basic paradigm of signaling described throughout this chapter, we will now discuss how it activates Protein Kinase G (PKG) a member of the Ser/Thr Protein Kinase AGC family.
The are two mammalian genes for PKG1 and PKG2. Both are homodimers. PKG1 acts in the cytoplasm while PKG2 becomes tethered to the membrane by N-terminal myristoylation. Figure \(\PageIndex{22}\) shows the domain structure of PKG-I, which is similar to PKG2.
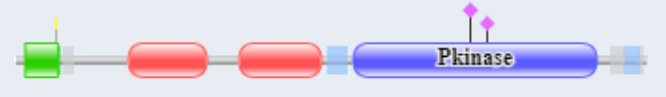
Red indicates the two nonidentical cGMP binding domains. Green is the N-terminal coiled-coil dimerization domain, which inhibits kinase activity in the absence of cGMP. On binding of cGMP, autoinhibition of the catalytic domain by the N-terminal domain is relieved. The binding of cGMP to the regulatory domain induces a conformational change which stops the inhibition of the catalytic core by the N-terminus and allows the phosphorylation of self (autophosphorylation) and then substrate proteins. Whereas PKG-I is predominantly localized in the cytoplasm, PKG-II is anchored to the plasma membrane by N-terminal myristoylation.
PKG1 is involved in modulating Ca2+ activity, platelet activation, smooth muscle contraction, gene expression as well as neural function. PKG2 helps regulate bone growth, intestinal secretion, and synaptic plasticity. It also regulates gene expression and activates the MAPK cascade in bone cells.
Figure \(\PageIndex{23}\) shows an interactive iCn3D model of the predicted structure of Human cGMP-dependent protein kinase 2 (AlphaFold, Q13237).
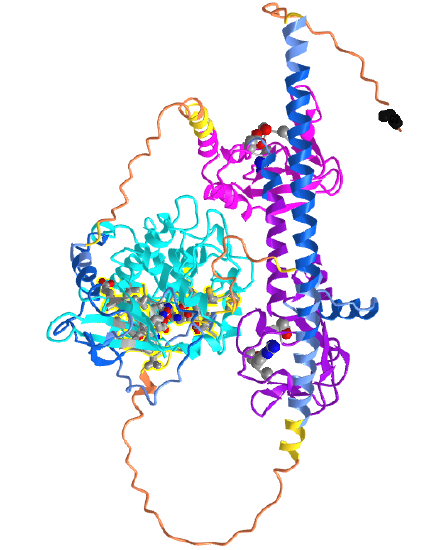
The cyan domain is the STK (Ser/Th Kinase) domain. The magenta and purple domains are the CAP-Ed domains. The spacefill side chain structures within them represent the cGMP (or with lower affinity cAMP) binding sites. The CPK-colored sticks in the cyan kinase domain are the amino acid side chains in the active site where ATP binds. The orange backbone represents the least confident part of the predicted structure. The black spacefill is the N-terminal Gly (after removal of Met) which is myristoylated, allowing targeting of the modified PKG2 to the cell membrane.



