28.5: Small G proteins, GAPs and GEFs
- Page ID
- 77358
Recent Updates - This section was extensively revised on 4/17/24
G proteins: Cellular Switch for Kinases
In the preceding chapter sections, we discussed two types of small G proteins, Gα, part of the heterotrimeric Gαβγ complex linked to GPCR signaling, and Ras (H, K, and N). Both bind GTP and GDP and have GTPase activity. When bound to GTP they are active while the GDP bound form is inactive. They have intrinsic GTPase activity, so eventually, active Ras will deactivate itself. What a perfect molecular switch to regulate signaling with this built-in off switch (the GTPase activity)! It turns out, however, that this simple on/off switch is too simple. For example, a single mutation that inhibits the GTPase activity would leave the protein on continually which could (and does) lead to unregulated growth and tumor formation. In addition, they are poor GTPases
To help with regulation, a new set of proteins called GAPs and GEFs modulate the balance of bound GTP (which activates the protein) and GDP (which inactivates the protein) in small G proteins. These are found abundantly in cells:
- GTPase activating protein or GAPs: As the name implies they enhance the intrinsic GTPase activity of the small G proteins, which would decrease G protein signaling;
- Guanine nucleotide exchange proteins or GEFs: These lead to the dissociation of bound GDP and its replacement with GTP, which would increase G protein signaling.
In addition, Ras is also regulated by changing its location in the cell. It is targeted to the cell and organalle membranes through the post-translational addition of a hydrophobic farnesyl and palmitoyl groups. At the membrane and when activated by GTP binding, active Ras can bind to and activate many proteins, most of which are protein kinases. Ras is just one member of a large superfamily of small G proteins, which all have GTPase activity.
Small G proteins
Small G proteins in the superfamily have a common 20 K molecular weight catalytic (GTPase) domain with 5 alpha helices, 6 beta strands, and connecting loops. Figure \(\PageIndex{1}\) shows the domain structures of the small G proteins called H-Ras as an example.
G boxes of the guanine (G)-binding domain are highlighted in orange. The hypervariable regThe P-loop, switch I, and switch II are colored green, red, and blue, respectively.
Let's describe Ras in more detail. Mammalian cells contain 3 variants of Ras, H-, N-, and K-, encoded by 4 genes, HRAS, NRAS, KRAS4A, and KRAS4B, with the two KRas forms arising by alternative splicing. They all bind GDP/GTP and have intrinsic but weak GTPase activity. Their amino acid sequences are shown below in Figure \(\PageIndex{2}\).
Figure \(\PageIndex{2}\): Christian W. Johnson, Derion Reid, Jillian A. Parker, Shores Salter, Ryan Knihtila, Petr Kuzmic, Carla Mattos, The small GTPases K-Ras, N-Ras, and H-Ras have distinct biochemical properties determined by allosteric effects, Journal of Biological Chemistry, 292, 2017, 12981-12993. ISSN 0021-9258, https://doi.org/10.1074/jbc.M117.778886. https://creativecommons.org/licenses/by/4.0/
Black denotes identical amino acids. Green amino acids in HRas (top row) show differences in at least one of the Ras proteins (isoforms). If the amino acid in HRas (top row) is the same in NRas, it is also shown in green in NRas (bottom row). Amino acids in NRas (bottom row) that are the same as KRas (above it) are shown in yellow. Unique amino acids in NRas (bottom row) are shown in cyan.
The five G "elements" (G1-G5) in the guanine binding G domain, found in all GTPases, are shown in red. G1 is the P-loop, G2 is switch I and G3 is switch II. G4 and G5 are important in binding the guanine base. These last G elements probably confer subtle allosteric changes in the structure that account for small differences in catalytic rates of GTP hydrolysis. Changing Lys117 or Asp 119 in the G4 domain decreases the affinity for guanine ligands and promotes dissociation.
Mutations at G1 (the P-loop) at amino acid G12 (G12C , G12S or G12V) and G13 (G13D), or in G3 (Switch II) at amino acid Q61 (Q61H) cause a large decrease in the GTPase activity of the protein, so they are continually in the "on" (constituitively-active) state, contributing to tumor formation. In mammals, these are the most common mutations found in tumors. In fact, Ras genes are mutated in around 20% of human cancers. About 75% of cancer patients with Ras mutations have mutated KRas while only 7% have mutated HRas. Which Ras gene is mutated depends on the tumor type. The activity of the Ras isoforms is often regulated by different GAPs and GEFs. Figure \(\PageIndex{3}\) shows the locations of the mutations associated with tumorigenesis.
Figure \(\PageIndex{3}\): Oncogenic mutation hot spots (red surfaces) highlighted in the GTP-bound RAS structure (gray cartoon, PDB 1QRA).. Kolch W, Berta D, Rosta E. Dynamic regulation of RAS and RAS signaling. Biochem J. 2023 Jan 13;480(1):1-23. doi: 10.1042/BCJ20220234. PMID: 36607281; PMCID: PMC9988006. Creative Commons Attribution License 4.0 (CC BY).
Mutations (K117N, K117R, and K147R) in the G4 and G5 domains can also be oncogenic not by decreasing the intrinsic GTPase activity but by lowering the affinity for G-nucleotides. This would increase the fractional saturation with GTP, which is more abundant in cells than GDP.
Figure \(\PageIndex{4}\) shows an interactive iCn3D model of human KRAS G12C mutant covalently bound to AMG 510, a covalent inhibitor (6OIM). This mutation flips the Ras switch so it is permanently on.
Figure \(\PageIndex{4}\): Human KRAS G12C covalently bound to AMG 510 (6OIM). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...Rx2ksMNmeCB7t6
The coloring coding is the same as in the above Figure 1:
- P-Loop (same as G1) is green, which is important for binding the phosphates in GTP/GDP;
- Switch 1 (30-40) is red and contains the invariant residue Thr 35;
- Switch 2 (60-76) is blue; Switch 1 and 2 are flexible loops that help sandwich GTP/GDP;
- The truncated C-terminal of the protein is shown in light pink (the end is very disordered so these amino acids do have clear electron density and are not found in the final crystal structure).
GDP is shown in colored sticks. The covalent inhibitor, AMG 510, is shown in spacefill and labeled MOV. It is covalently attached to the Cys 12 in the mutated version.
The G12C human KRas mutation is found in about 13% of patients with non-small cell lung cancer (NSCLC) which is any type of epithelial lung cancer that is not small cell lung cancer. NSCLCs include squamous cell carcinoma, large cell carcinoma, and adenocarcinoma. Before the discovery of AMG 510, Ras was considered "undruggable" since it was devoid of obvious pockets for drug inhibitors. The G12C mutation replaces glycine with cysteine, a potent nucleophile that can react with the covalent inhibitor and inhibit the constitutively-active protein. A new generation of inhibitors attempts to modify different aspects of Ras activity, including its localization to the membrane, the binding of different effectors, and nucleotide exchange.
The structure above lacks the last 20 amino acids of the C terminal end with the polybasic region and the C-terminal CAAX of the hypervariable region that especially differentiates Ras from other small G-proteins. The last residues of K-Ras from 180-186 are CVKIKKCIIM. C180 and the K side chains are post-translationally palmitoylated while C186 is farnesylated. These post-translational lipidations target the otherwise soluble enzyme to the membrane. Figure \(\PageIndex{5}\) below shows a model of K-Ras targeted to the intracellular side of the bilayer (two sets of black "dummy" atoms represent lipid molecules) through the attachment of a farnesyl group on Cys185. Cys-farnesyl 185 is shown in spacefill.
Figure \(\PageIndex{5}\): A model of K-Ras targeted to the intracellular side of the bilayer (two sets of black "dummy" atoms representing lipid molecules) through the attachment of a farnesyl group on Cys185. Cys-farnesyl 185 is shown in spacefill. The full-length structure was obtained through NMR spectroscopy (7KYZ, Model 1) and added to PC bilayer (represented by dummy atoms) using MolCube.
Indeed, all Ras proteins are localized to different membranes (plasma and organelle) through post-translational addition of hydrophobic anchors to the C-terminal hypervariable region. They can be activated at different rates at the plasma, endoplasmic reticulum and Golgi membranes so the downstream signaling from Ras likely emanates in waves from different locations in the cell.
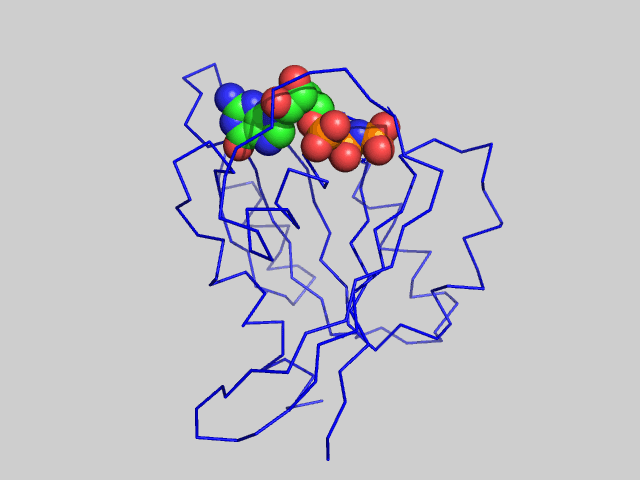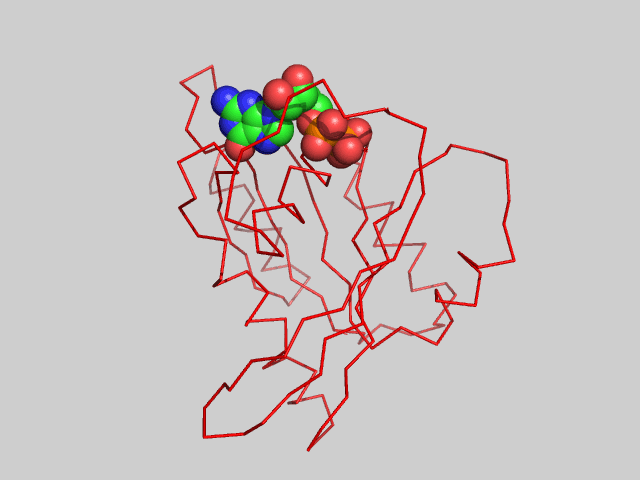
Figure \(\PageIndex{6}\) is an animation showing structural differences between the GTP bound form (blue, pdb id 5p21) and GDP form (red, pdb id 4q21) of the H-Ras protein. One helix and nearby loops are perturbed, especially Switch I.
There are about 150 members of the human Ras superfamily as shown in Figure \(\PageIndex{7}\) and Table 1 below. They are divided into Ras, Rho, Rab, Arf, Ran and "other" subfamilies.
Table \(\PageIndex{7}\) shows common Ras superfamily functions
| Ras | regulation of gene expression, cell proliferation, survival, and differentiation |
| Rho | regulation of actin cytoskeleton, cell shape, and movement, cell interactions with the extracellular matrix |
| Rab | vesicle trafficking, endocytosis, secretion |
| Arf | vesicle trafficking, endocytosis, secretion, microtubule assembly |
| Ran | nuclear cytoplasm transport, mitotic spindle |
Regulation of small G proteins: GAPs and GEFs
Given the critical importance of small G proteins, it makes biological sense that their on/off activity would be exquisitely regulated. Indeed, they are. Two families of proteins have evolved to regulate them by determining whether GTP or GDP is bound to the protein (leading to an active, and inactive small G protein respectively). One family, GTPase activating proteins (GAPs) facilitate the hydrolysis of bound GTP, leading to the inhibition of the protein. The other family are GTP exchange proteins (GEFs), which facilitate the exchange of GTP for GDP, activating the protein.
The activity of Ras GAPs and GEFs, as well as various proteins interacting with Ras, are depicted in Figure \(\PageIndex{8}\).
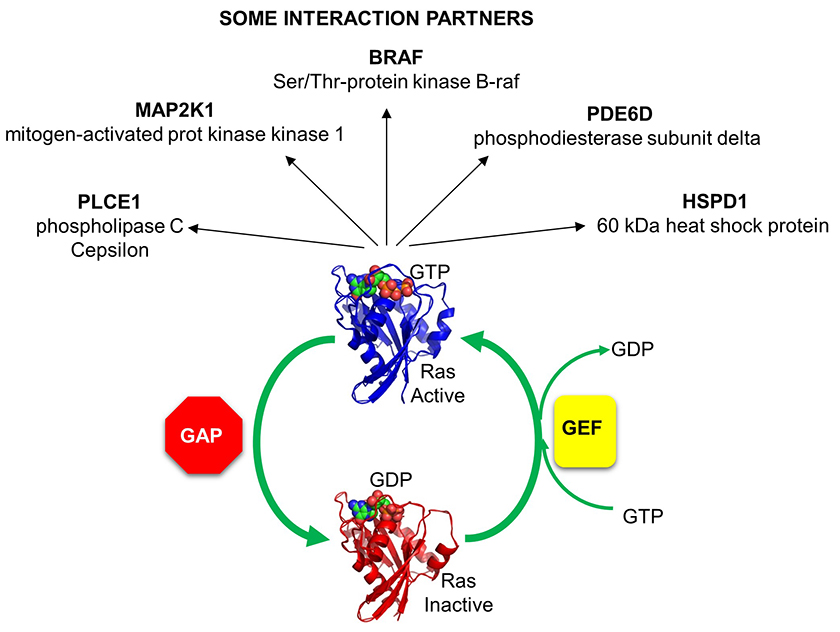
Figure \(\PageIndex{8}\): The activity of Ras GAPs and GEFs, as well as various proteins interacting with Ras
It may seem crazy but the number of GEFs and GAPs is greater than the number of small G proteins. As shown in Figure \(\PageIndex{4}\), there are 20 Rho G proteins but about 80 GEFs and 70 GAPs for them. This number presumably allows greater control of the specificity of the reactions controlled by the Rho G protein.
GAPs - GTPase Activating Proteins
The hydrolysis of the gamma phosphate of GTP by water in Ras proceeds by a pentavalent transition state with two axial and three equatorial ligands to the phosphorous. Developing charge in the transition state would usually be stabilized by catalytic residues in the catalytic domain of Ras. However, Ras is a poor GTPase. That's where GAPs comes in. In the Ras/GAP complex, GAP positions its Arg 789 on the GAP in a position to stabilize the transition state for Ras-bound GTP cleavage. This Arg 789 is almost in the same position as Arg 178 in the Galpha inhibitor subunit of a heterotrimeric G protein which inhibits GPCR signaling. Both of these arginines have similar catalytic functions.
Figure \(\PageIndex{9}\) shows an interactive iCn3D model of Ras-GAP complex (1WQ1)
.png?revision=1&size=bestfit&width=509&height=375)
Ras is shown in secondary structure colors, while GAP is in gray. GDP-AlF3, a GTP analog, is shown in color spacefill. Arginine 789 in GAP is shown in spacefill with CPK colors and labeled R789. It is in position to stabilize the bound GTP in the complex and its cleavage transition state.
GEFs - GTP Exchange Factor
Once bound to Ras, GDP dissociates very slowly. Values of 10-5 sec-1 have been reported for the first-order rate constant of the dissociation of GDP from a small G protein in an isolated syste. Assuming that the diffusion controls the on-rate constant for the complex, the dissociation constant KD=koff/kon for the G protein:GDP complex would be 0.1 pM and its half-life would be 0.8 days, similar to the lac repressor:DNA operator complex. Hence the protein, when bound to GDP, is essentially locked in the off position. What if it needs to be reactivated quickly? How can the rate at which GDP dissociates be increased so that GTP could replace it? If it were to dissociate, GTP could quickly replace it based on simple equilibrium conditions as Ras since the GTP concentration is much higher in cells than GDP.
One could envision several ways to change the rate at which GDP dissociates. In organic chemistry, a favorite student response to many questions is to evoke steric effects. In biochemistry, the analog is often conformational changes. How could you change the conformation of Ras such that it might favor GTP binding and GTP association? That could occur by conformational changes in Ras associated with ligand binding or more likely by a post-translation modification such as phosphorylation as part of a signaling process. For small G proteins, another mechanism is evoked: the binding of another protein, a GTP Exchange Factor or a GEF, which promotes GTP exchange for the bound GDP. If the Ras:GEF:GDP complex has a 10,000 increase in koff for GTP, the half-life of the bound GDP is 7 seconds. There are 80 GEFs in the human genome. If you think about it, in GPCR coupled signaling, the ligand-bound GPCR is a GEF for the Gα subunit of the heterotrimeric Gαβγ protein.
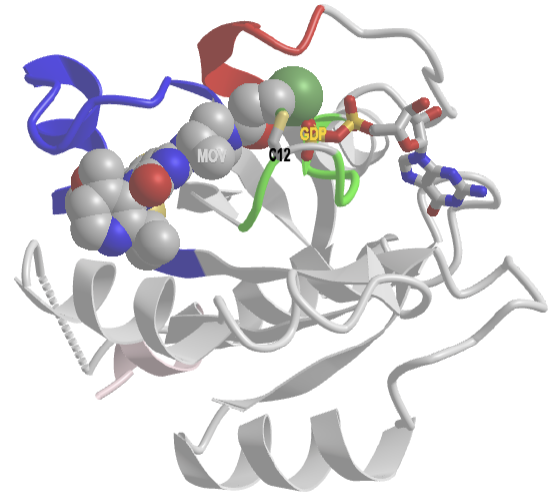
The crystal structure of a Ras GEF, SOS, in complex with Ras allows a detailed understanding of the mechanism. SOS (son of sevenless), a cytoplasmic protein, is recruited to the cell membrane where active Ras is found, tethered to the membrane with a hydrophobic farnesyl attachment. Figure \(\PageIndex{10}\) shows an interactive iCn3D model of H-Ras and SOS (a GEF) complex (1bkd).
Figure \(\PageIndex{10}\): Ras and SOS (a GEF) complex (1bkd) (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...XJP4Fajqc9eEL6
SOS is shown in cyan, while H-Ras is shown in gray. As in the figures above, the P-Loop (same as G1) is green,, Switch 1 (30-40) is red and contains the invariant residue Thr 35 and Switch 2 (60-76) is blue.
The actual biological unit (functional structure) is a hetero 8-mer (A4B4) with C4 symmetry. The iCn3D model shows just a heterodimer for clarity. SOS as a GEF affects nucleotide binding to Ras in two essential ways. An alpha helix from SOS displaces Switch 1 (amino acids 30-38, shown in red) in Ras, which opens the binding site for the guanine nucleotides. Additional conformational changes in Switch II (59–72, blue) in Ras and interference from the side chains from the SOS alpha helix interfere with the binding of the phosphates on the bound nucleotide. This promotes the dissociation of the bound nucleotide and Mg2+. Now GTP can preferentially rebind. How?
Before we answer that question, let's explore the conformational differences just in the structure of Ras in the Ras:GDP complex (4q21) and Ras:GTP analog (6q21) These differences are shown in Figure \(\PageIndex{11}\). The light gray is Ras:GDP and the dark gray is Ras:GCP (a GTP analog - the larger spacefill molecule)). The red backbone is Switch 1 and the blue is Switch 2.
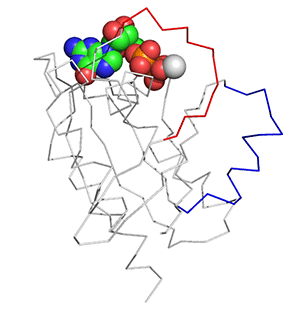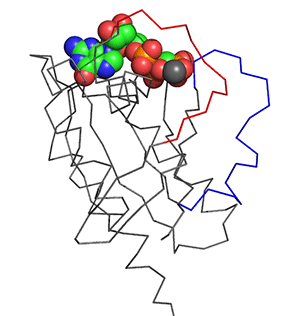
Figure \(\PageIndex{11}\): Conformational differences just in the structure of Ras in the Ras:GDP complex (4q21) and Ras:GTP analog (6q21)
Now let's look at the difference between Ras in the Ras:GDP complex (4q21) and Ras in the Ras:SOS (1bkd) complex. These differences are shown in Figure \(\PageIndex{12}\). The light gray backbone is Ras:GDP alone, with the GDP shown in CPK colors and in spacefill. The dark gray backbone depicts just the Ras protein from the Ras:SOS protein complex. In both, the red backbone is Switch I and the blue backbone is Switch 2.
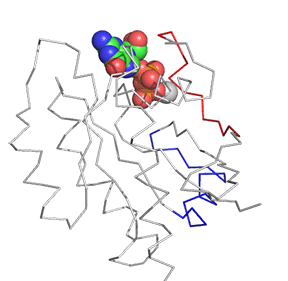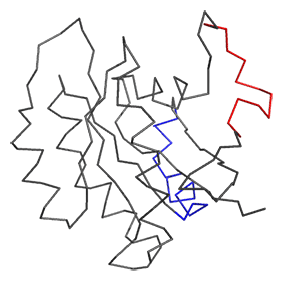
Note the large shift in Switch 1 in the Ras structure going from the Ras:GDP to the Ras in the Ras:SOS complex. This leaves a "gaping" hole from which GDP can "escape". Now how do the opening of the active site and release of bound GDP facilitate GTP binding?
The conformational changes on RAS binding to SOS open up the active site, allowing GDP dissociation. GTP can now replace it since from an equilibrium point of view, Ras and other small G proteins favor GTP binding since the concentration of GTP in the cell is higher than that of GDP. In addition, some additional noncovalent interactions with the extra phosphate on GTP probably help.
Figure \(\PageIndex{13}\) shows a cartoon showing the changes in Ras on GEF binding as illustrated in these coupled chemical equilibria:
GEFloose:Ras:GDPtight ↔ GEFtight:Ras:GDPloose ↔ GEFtight:Ras
Ras Activation and Signaling
Upstream activation of Ras
Now let's place Ras activity in the context of large signaling pathways in which it partakes. Just a note to beginning students of signal transduction, who might default to thinking that every signaling protein is a membrane receptor, a kinase, a phosphatase, or another protein that causes post-translational modification of other proteins. Ras is none of these. It's just a very small globular protein that can cleave bound GTP and does so quite poorly. Yet it lies at the center of the control of many signaling pathways, and when it gets dysregulated, cancer often arises.
First, signaling by Ras requires it to be associated with membranes or membrane-less cytoplasmic protein granules. Targeting of Ras to membranes requires its lipidation (palmitoylation, farnesylation) in the hypervariable region and a host of enzymes and other proteins. We won't discuss those or their regulation. We will start with nonactive Ras associated with membranes. Figure \(\PageIndex{14}\) summarizes Ras:membrane interactions.
Figure \(\PageIndex{14}\): Busquets-Hernández C, Triola G. Palmitoylation as a Key Regulator of Ras Localization and Function. Front Mol Biosci. 2021 Mar 17;8:659861. doi: 10.3389/fmolb.2021.659861. PMID: 33816563; PMCID: PMC8010249. Creative Commons Attribution License (CC BY)
Panel (A) The Ras isoforms contain a highly homologous G domain (90%) and a C-terminal hypervariable region (HVR) that comprises the last 24 amino acids. The HVR exhibit a low degree of homology between isoforms (∼ 10%) and present different post-translational lipid modifications. Red cysteines (C) are farnesylated, green cysteines (C) are palmitoylated, blue lysines (K) are polybasic residues.
Panel (B) Ras membrane distribution is dynamic and depends on membrane targeting motifs (polybasic sequences and lipids) and changes in palmitoylation state that combined, modulate Ras trafficking and localization to specific membranes (PM, endomembranes, subdomains). Hence, farnesylated H/N-Ras get palmitoylated at the Golgi apparatus by DHHC9/Golga7 and then are transferred to the PM via the secretory pathway. After depalmitoylation, H/N-Ras traffic back to the Golgi to be reacylated. Due to the presence of two palmitoyl moieties H-Ras gets enriched in the PM, whereas N-Ras is predominantly localized at the Golgi. Once in the membrane, H-Ras segregates in different microdomains (rafts, non-rafts) in a GDP/GTP-dependent manner. Palmitoylated N-Ras associates preferentially with raft/non-raft boundary regions, although a N-Ras protein modified with an unsaturated palmitoleic shows preferential accumulation in fluid domains. The localization of K-Ras4A on the PM relies on the presence of polybasic regions and a palmitoylated cystine, whereas K-Ras4B is palmitoylation independent. After depalmitoylation, K-Ras4A travels to the mitochondria and binds HK1.
Once at membranes, the rate of Ras activation is influenced by three factors:
- the rate of bound GTP hydrolysis
- the rate of GTP/GDP binding and exchange
- the rate of binding downstream effect proteins (GAPs and GEFs) that regulate its activity
Let's look at one concrete example that we described in the previous section (28.4), signaling through the epidermal growth factor receptor (EGFR). When the receptor binds the extracellular signal (epidermal growth factor), it dimerizes. The intracellular domains of the now dimeric EGFR autophosphorylates themselves on selected tyrosine side chains. This then recruits a protein called Growth factor receptor-bound protein 2 (GRB2), which has an SH2 (Sarc or Src Homology 2) domain that binds phosphotyrosine motifs in proteins. GRB2 acts as an adaptor protein in that in addition to the SH2 domain, it has two SH3 (Sarc or Src Homology 3) domains that bind proline-rich domains on other signaling proteins, including the protein Sos of sevenless homolog (SOS), a major GEF that we discussed above. GRB2 does not have enzymatic activity. SOS then interacts with the membrane-associated Ras. These events are illustrated in Figure \(\PageIndex{15}\) below.
Figure \(\PageIndex{15}\): Near downstream signaling molecules after activation of EGFR. Mattox TE, Chen X, Maxuitenko YY, Keeton AB, Piazza GA. Exploiting RAS Nucleotide Cycling as a Strategy for Drugging RAS-Driven Cancers. Int J Mol Sci. 2019 Dec 24;21(1):141. doi: 10.3390/ijms21010141. PMID: 31878223; PMCID: PMC6982188. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Downstream activation by Ras
When membrane-bound Ras interacts with the GEF SOS, it is activated. Active Ras then can activate a plethora of other downstream signaling molecules by binding to them and altering their activity allosterically (remember Ras is NOT a kinase or other PTM agent). Figure \(\PageIndex{16}\) illustrates 12 classes of downstream signaling proteins activated by Ras. We'll explore some of them in future chapter sections.
Figure \(\PageIndex{16}\): RAS activation cycle and RAS effectors. Fifty-six bona fide RAS effectors can be grouped into 12 classes according to sequence homology and regulation of downstream biochemical and biological processes. Kolch W, Berta D, Rosta E. Ibid.
We'll focus briefly on just two downstream proteins, RAF1 kinase and EGFR (which we discussed briefly above). For many years nobody knew how Ras activated signaling. That changed when it was discovered that Ras binds RAF1 kinase. RAF1 contains three conserved regions, CR1, CR2 and CR3 (which is the kinase domain). CR1 contains the Ras binding domain (RBD) for Ras and a cysteine rich domain (CRD) that interacts with membrane bilayers. Figure \(\PageIndex{18}\) shows the domain structures of RAF1 and KRas, along with a truncated construct of Raf1 that contains just the RBD connected by a linker to the CRD of Raf1. Using surface plasmon resonance experiments (shown in panel B), it is clear that the binding of the RBD-CRD segment occurs with a higher affinity/lower KD (=ligand concentration at half-maximal saturation) than just the RBD segment.
Figure \(\PageIndex{18}\): Structure of the KRAS-RAF1(RBDCRD) complex and SPR analysis. Tran, T.H., Chan, A.H., Young, L.C. et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat Commun 12, 1176 (2021). https://doi.org/10.1038/s41467-021-21422-x. Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/.
Panel a: Domain architecture of RAF1 and KRAS. Key domains and their boundaries are shown. The RAF1 construct containing RBD and CRD regions used in this study is shown below the full-length RAF1. The G-domain of KRAS4b(1–169) used in this study contains switch-I (S-I) and switch-II (S-II) regions with the inter-switch region present between them.
Panel b: Steady-state binding isotherms derived from the representative SPR experiments used for measuring the binding affinities of RAF1(RBDCRD) and RAF1(RBD) to KRAS-GMPPNP. Source data are provided as a Source Data file.
Panels c, d: The overall structure of the complex formed by GMPPNP-bound KRAS and RAF1(RBDCRD) in c cartoon, and d surface representations. GMPPNP is shown as sticks, and Mg2+ (green) and Zn2+ (gray) are shown as spheres. KRAS is colored pink with switch-I and switch-II regions highlighted in magenta and salmon, respectively. RBD, CRD, and the linker present between them are colored cyan, green and yellow, respectively.
Figure \(\PageIndex{19}\) shows a model of the CRD-loop-RBD construct from Rak1 and KRas interacting with each other and a membrane bilayer.
Figure \(\PageIndex{19}\): Membrane interacting residues in CRD, and a model showing the crystal structure of KRAS-RBDCRD at the membrane. Tran, T.H., Chan, A.H., Young, L.C. et al. Ibid.
Crystallographic KRAS-RBDCRD oriented with KRAS helices α4 and α5 in membrane contact, as observed in MD simulations of KRAS28. Lipids are sticks with spheres for headgroup phosphorus atoms; KRAS-RBDCRD has the same color-coding as the preceeding figure.
Side chains of CRD residues 143RKTFLKLAF151 and 157KFLLNGFR164 are sticks with purple carbon atoms. The interaction of helices 4 and 5 in KRas leads to insertion of two CRD loops (143RKTFLKLAF151 and 157KFLLNGFR164 ) into the membrane.
Figure \(\PageIndex{20}\) offers a model that shows that binding of an autoinhibited monomer of Raf1 to membrane-associated KRas leads to conformational changes within Raf1 and relief of autoinhibition, allowing Raf1 to dimerize with another Raf (in this case BRaf) also bound to an adjacent membrane-associated KRas. Before binding to KRas, Raf1 is bound to the dimeric protein 14-3-3 (an adapter protein in some signaling pathways) which interacts with "phosphorylated serines present in CR2 and CR3, keeping RAF in an autoinhibited state."
Figure \(\PageIndex{20}\): Membrane interacting residues in CRD, and a model showing the crystal structure of KRAS-RBDCRD at the membrane. Tran, T.H., Chan, A.H., Young, L.C. et al. Ibid.
Crystallographic KRAS-RBDCRD is oriented with KRAS helices α4 and α5 in membrane contact, as observed in MD simulations of KRAS. Lipids are sticks with spheres for headgroup phosphorus atoms; Side chains of CRD residues 143RKTFLKLAF151 and 157KFLLNGFR164 are sticks with purple carbon atoms. Effectively, the interaction between Ras and Raf leads to a dimerization of both Ras and Raf, leading to the activation of Raf.
Further simulations show that additional Ras proteins associate to form nanocluster of 6-8 Ras proteins at the membrane. This is illustrated in the bottom left of Figure \(\PageIndex{17}\) below (although the number of Ras monomers is likely overrepresented).
Figure \(\PageIndex{17}\): Ras signaling pathways. Ras forms nanoclusters and promotes Raf dimerization in the Raf/MEK/ERK (MAPK) pathway (lower left). Nussinov R, Tsai C-J and Jang H (2019) Does Ras Activate Raf and PI3K Allosterically? Front. Oncol. 9:1231. doi: 10.3389/fonc.2019.01231. Creative Commons Attribution License (CC BY)
A similar mechanism occurs for the the activation of the membrane-associated kinase PI3Kα by Ras as illustrated in the middle of the figure above (this is also described somewhat in Figure 15). The autoinhibited (or latent) form of PI3Kα converts to an active form on binding to the autophosphorylated cytoplasmic domain of the EGFR. Ras doesn't allosterically lead to the activation of PI3Kα per se, but when bound to PI3Kα, Ras keeps the active PI3Kα at the membrane longer for prolonged signaling.
Post-translational Modification of Ras
You might have guessed that Ras can be further regulated by post-translational modifications (other than lipidation). Two examples are shown in Figure \(\PageIndex{21}\) below.
Figure \(\PageIndex{21}\): RAS regulation by posttranslational modifications.
Panel (A): Protein kinase C (PKC) phosphorylation of KRas at S181 in the polybasic region (lowering the net positive charge on that region). This keeps KRas activated by preventing GAP binding. It also induces mitochondrial translocation of KRas, where it promotes apoptosis by binding to and inhibiting the function of the anti-apoptotic Bcl-XL protein.
Panel (B): SRC (a protein kinase) can also phosphorylate tyrosines 32 and 64. This inhibits Ras by interfering with RAF binding and promoting association with GAPs. Kolch W, Berta D, Rosta E. Ibid.
Before leaving this chapter section, let's review one more small G protein, Ran. Ran acts as a mediator of protein movement across the nuclear membrane (we discussed this in Chapter 11.5). It's mainly in the GDP-bound form in the cytoplasm and the GTP-bound form in the nucleus. It switches between a cytoplasmic GDP- and a nuclear GTP-bound state by nucleotide exchange and GTP hydrolysis. Nuclear import receptors with bound cargo protein containing a nuclear import signal bind RAN-GTP in the nucleus, leading to the release of importin and the cargo protein. In contrast, cargo proteins with a nuclear export signal bind exportins and RAN-GTP in the nucleus and move into the cytoplasm, where the RAN-bound GTP is hydrolyzed on binding a RAN-GAP. This cycle is illustrated in Figure \(\PageIndex{22}\).
Figure \(\PageIndex{22}\): Model of nuclear import and export. Cargo containing NLS (Nuclear localization signal) is imported with the help of Importin α and importin β heterodimer. Nuclear export of cargo having NES (nuclear export signal) is carried out with the help of exportins. Ran GTP is also required during that process. Khan Asmat Ullah, Qu Rongmei, Ouyang Jun, Dai Jingxing. Front. Physiol., 03 April 2020 | https://doi.org/10.3389/fphys.2020.00239. Creative Commons Attribution License (CC BY).




_complex_(1bkd)4.png?revision=1&size=bestfit&height=370)