32.4: Biofuels A - Corn and Sugar Cane Ethanol
- Page ID
- 98020
Introduction
The world has a great need for energy. We have invested vast sums of money in finding and using fossil fuels. Fossil fuels seem to be an ideal energy source since they are highly reduced, easily stored, energy-dense, and highly abundant. Yet we now know the immense cost of their use: pollution that shortens lives and climate change. We have dramatically increased our bioethanol production from corn and sugar cane to remove our reliance on fossil fuels for transportation. Ethanol is partially oxidized as it has one oxygen atom in the two-carbon molecule. Hence, the energy released per gram is about 63% (by mass) and 70% (by volume) compared to gasoline. The energy values for various fuels are shown in Table \(\PageIndex{1}\) below, where ΔHc° is the standard enthalpy of combustion.
|
Name |
Formula |
State |
-ΔHc° |
-ΔHc° |
-ΔHc° |
|---|---|---|---|---|---|
|
Ammonia |
NH3 |
gas |
383 |
22.48 |
5369 |
|
Butane |
C4H10 |
gas |
2878 |
49.50 |
11823 |
|
Carbon (graphite) |
C |
cry |
394 |
32.81 |
7836 |
|
Carbon monoxide |
CO |
gas |
283 |
10.10 |
2413 |
|
Ethanol |
C2H6O |
liq |
1367 |
29.67 |
7086 |
|
Hydrogen |
H2 |
gas |
286 |
141.58 |
33817 |
| Methane | CH4 | gas | 891 | 55.51 | 13259 |
|
Methanol |
CH4O |
liq |
726 |
22.65 |
5410 |
|
Naphthalene |
C10H8 |
cry |
5157 |
40.23 |
9609 |
|
Octane |
C8H18 |
liq |
5470 |
47.87 |
11434 |
|
Propane |
C3H8 |
gas |
2220 |
50.33 |
12021 |
| wood (red oak) | 14.8 | 3540 | |||
| coal (lignite) | 15 | 3590 | |||
| coal (anthracite) | 27 | 4060 | |||
| methyl stearate (biodiesel) |
(CH3(CH2)16(CO)CH3 | 40 | 9560 |
Nevertheless, ethanol is readily made and is a valuable biofuel. A glance at the table suggests that H2 would be the best possible fuel, given that it has the highest energy release per gram and contains no carbon. At present, it can't be produced at the scale needed, and it isn't easy to store and transport. The critical infrastructure for its widespread use is lacking. Yet these factors could be solved. We'll explore biohydrogen in a separate chapter section.
In theory production of ethanol from plants at first glance is carbon neutral since each carbon in the ethanol is fixed from atmospheric CO2 during photosynthesis. Combustion of ethanol then returns the CO2 to the atmosphere in a net zero emission fashion, as shown in the reaction below.
6CO2 (g) + 6H2O (l) → C6H12O6 (s) + 6O2 (photosynthesis)
C6H12O6 (s) → 2 CH3CH2OH (l) + 2CO2 (g) (anaerobic ethanol biosynthesis)
2CH3CH2OH (l) + 6O2 → 4CO2 (g) + 6H2O (g) (combustion of ethanol)
Six CO2s in, six out! It seems simple but it's not. We'll explain more later. First, let's explore how ethanol is synthesized for its two major uses, drinking and use as a biofuel.
Ethanol Production Overview
The scale of worldwide ethanol production is quite staggering. Let's first consider the production of ethanol by yeast for alcoholic beverages. About 100 billion US gallons/yr (BGY) of beer, 7 BGY of wine, and 6 BGY of spirits are produced yearly. Assuming beer, wine and spirits are about 5%, 12%, and 40% percent ethanol by volume, respectively, the volume of actual ethanol/year made by yeast in these alcoholic beverages is about 5 BGY (beer), 0.85 BGY (wine) and 2.4 BGY (spirits). This sums to about 8.3 billion gallons of ethanol produced by these microorganisms. Compare this to fuel ethanol production each year, shown in Figure \(\PageIndex{1}\).
Figure \(\PageIndex{1}\): US Fuel Ethanol Production. Data from U.S. Bioenergy Statistics
Note that the y-axis is in units of 1000s gallons of ethanol. Peak US production was in 2018, when 16 billion gallons were produced, about 1/10 of the gasoline used yearly in the US. The year Renewable Fuel Standards (RFS) were introduced in the USA (2005) is also shown. This dip in 2020 is attributed to the COVID pandemic.
Combined, the US and Brazil produce about 85% of fuel ethanol, as shown below in Figure \(\PageIndex{2}\).
Figure \(\PageIndex{2}\): Fuel ethanol production (billions of gallons or BG) around the world per year. https://afdc.energy.gov/data/10331
Almost all US ethanol is made from corn, while Brazil's primary source is sugar cane.
Since the significant ramp-up of fuel ethanol around 2005, the world now produces 3x the amount of ethanol to drive our outsized vehicles than microorganisms have for our drinking. These statistics show that the world can quickly respond when it meets our needs.
An overview of ethanol biosynthesis
Whether ethanol is made for the beverage or biofuel industries, yeast play the major role, as we explored in Chapter 14.2: Fates of Pyruvate under Anaerobic Conditions- Fermentation. Yeast contains all the enzymes necessary to convert glucose (6C), made from various "feedstocks", to pyruvate (3C) through the glycolytic pathway. This is followed by the conversion of pyruvate to ethanol using two key yeast enzymes. First, pyruvate is decarboxylated to acetaldehyde by pyruvate decarboxylase, which uses TPP as a cofactor. Acetaldehyde is then reduced to ethanol by ethanol dehydrogenase, using NADH as a substrate, in a process that reforms NAD+, allowing glycolysis to continue. These combined anaerobic reactions, known as fermentation, are shown in Figure \(\PageIndex{3}\).
Figure \(\PageIndex{3}\): Summary of Ethanol Fermentation in Yeast
Yeast are facultative (not obligate) anaerobes in that they can produce energy by glycolysis and ethanol fermentation in the absence of oxygen. Of course, in the presence of oxygen, the pyruvate made from glycolysis in yeast is preferentially converted to acetyl-CoA, which enters the citric acid cycle and oxidative phosphorylation pathways to maximize ATP production. Yeast is abundant, so all that is needed is a large source of glucose.
An abundant source of glucose for bioethanol production are plants that contain starch (for example corn) or abundant simple sugars (for example sucrose in sugar cane). Starch, an α (1,4) polymer of glucose with α (1,6) branches, can easily be broken down in an industrial setting with amylases to form glucose. A significant problem with this "first" generation source of glucose is that food crops (corn, and to a lesser degree sugar cane) are used for biofuel consumption instead of for food. "Second" generation sources of glucose are crop and wood waste products that contain cellulose, a β (1,4) polymer of glucose which is found with another carbohydrate polymer lignin. A significant problem with the use of cellulose is the high chemical stability of the β (1,4) glycosidic bond. Fungi and bacteria are sources of β-glycosidases to liberate free glucose from cellulose. "Third" generation sources of glucose use algae, which does not displace cropland for bioethanol production. A "fourth" generation source of glucose are genetically engineered organisms, which might become future sources of bioethanol. Figure \(\PageIndex{4}\) summarizes the different generations of feedstock sources for bioethanol production.
Figure \(\PageIndex{4}\): Generation feedstock sources for bioethanol production. Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Fermentation 2021, 7, 268. https://doi.org/10.3390/fermentation7040268. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
We will discuss the use of first generation sources, corn and sugar cane, which are used to produce most of the world's bioethanol, in this chapter section, and the other two in subsequent sections.
Corn Bioethanol
Corn is a significant source of starch, an α (1,4) polymer of glucose with α (1,6) branches. Hence glucosidases are used to hydrolyze starch to glucose. First, the dry corn is ground in a mill, breaking the outer coat of the corn kernel and increasing access to the starch. Heated water is added to form a mash or slurry. Cooking at greater than 85o C helps hydrolyze some glycosidic bonds and lowers the viscosity of the slurry. In the process of liquification, the pH is adjusted to around 6.0. Different α-amylases (endoglycosidases) are added, which cleave the α (1,4) glycosidic bonds to produce shorter dextrins (containing branched glucose units not cleaved by alpha-amylases), and α (1,4) linked glucose oligosaccharides of lengths from 2 glucose units (called maltose) up to 7-8. β-amylase, an exoamylase, is also used, which successively cleaves maltose units, Glc α (1,4)Glc, from the nonreducing ends of the chains
Alpha-amylases
A mixed-rendered structure of the human pancreatic alpha-amylase is shown below in Figure \(\PageIndex{5}\).
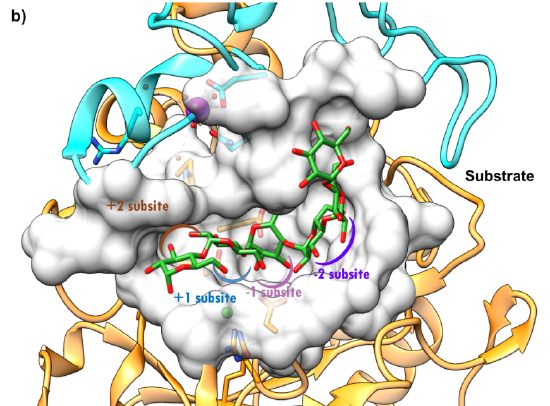
Figure \(\PageIndex{5}\): Surface representation of the active site of HPA (5TD4) https://pdb101.rcsb.org/global-healt...ha-glucosidase. CC-BY-4.0 license. Attribution: David S. Goodsell and the RCSB PDB.
The surface view highlights the deep C-shaped groove into which the substrate, in this case, octaose, is bound. Consistent with substrate numbering for proteases, the starch substrate is numbered ..-2, -1, +1, +2, with cleavage occurring between the -1 and the +1 bound alpha-glucose residues. The protein has three domains (orange, blue, and pink). This particular structure had an active site mutant (Asp300Asn, D300N). The enzyme has bound calcium and chloride ions. Ca2+ maintains the necessary structure, while Cl-, bound in the C domain, is an allosteric activator.
The octaose binding site is between the A and B domains. Asp197, Glu233, and Asp300 are critical catalytic residues, with Asp 197 acting as a nucleophile to produce a glycosylated intermediate, which is hydrolyzed in the next step. Asp197 and Glu233 act as general acids/bases. We will explore in depth similar mechanisms for the action of beta-amylase (below) and cellulase (next chapter section).
Figure \(\PageIndex{6}\) shows an interactive iCn3D model of starch binding sites on the Human pancreatic alpha-amylase D300N variant complexed with an octaose substrate (5TD4)
Figure \(\PageIndex{6}\): Starch binding sites on the Human pancreatic alpha-amylase D300N variant complexed with an octaose substrate (5TD4). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...W29jf4yAc1JEq9
The domains in the enzyme are colored-coded, as in Figure 4. Key active site residues for binding and catalysis are shown as sticks and labeled.
Beta amylase
β-amylase (also called β-1,4-maltosidase) is a key enzyme in the saccharification process, in which starch and cellulose are broken down into monosaccharides. β-amylase is abundant in crops (wheat, barley, soybeans, etc.) and other higher plants, as well as bacilli and fungi. It is used in making beer and caramel (malt syrup). As an exo-glycosidase, it cleaves Glc α(1,4) Glc (maltose) units from the nonreducing end of starch. It is called β-amylase since the hydrolysis proceeds with the inversion of configuration at the reducing end of the freed maltose. It can't cleave at α-1,6 branches, so if used alone, this enzyme produces free maltose and large β-limit dextrins. When fruits ripen, the enzyme cleaves starch to produce sweet maltose. It is also used in seed germination.
Plants have to sprout, which requires energy and free sugars. Maltose is produced on activation of β-amylase during seed germination and sprouting. Although maltose is less sweet than sucrose and fructose, it is used in hard candies, given its tolerance to the heat needed in candy production. Malting of grains is accomplished by adding water to sprout them, leading to maltose and other sugars forming. This is followed by drying, with the malted grains used as sweeteners in the food industry. Malted grains are used to produce beer, whisky, some baked goods, and drinks. Barley is the most commonly malted grain used in cereals.
Huge amounts of amylases are needed for corn ethanol production, and they must withstand the conditions necessary for the industrial production of ethanol. Much effort has been devoted to finding and characterizing microbial β-amylases. We'll describe one, AmyBa, from B. aryabhattai. Figure \(\PageIndex{7}\) shows sequence similarities among various bacterial β-amylases.
Figure \(\PageIndex{7}\): Sequence and structure analysis of AmyBa. . Duan, X., Zhu, Q., Zhang, X. et al. Expression, biochemical and structural characterization of high-specific-activity β-amylase from Bacillus aryabhattai GEL-09 for application in starch hydrolysis. Microb Cell Fact 20, 182 (2021). https://doi.org/10.1186/s12934-021-01649-5. Creative Commons Attribution 4.0 International License. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
Panel A shows multiple sequence alignments of β-amylases. The strictly conserved residues are displayed on a red background, and the highly conserved residues are shown on a yellow background. The secondary structure elements are shown for B. cereus β-amylase (PDB ID: 5BCA). The signal-peptide-cleavage site and two catalytic residues (E) are indicated by black triangles (black inverted triangles). Conservation of the flexible loop motif (HXCGGNVGD) is noted. β-amylase accession numbers are as follows: B. aryabhattai (WP_033580731.1), B. cereus (P36924.2), B. flexus (RIV10038.1), B. firmus (P96513.1), B. circulans (P06547.1), T. thermosulfurigenes (P19584.1).
A comparison of the structures of B. aryabhattai β-amylase with soybean β-amylases is shown in Figure \(\PageIndex{8}\).
Figure \(\PageIndex{8}\) B Three-dimensional molecular model of B. aryabhattai β-amylase (AmyBa). C Superimposition of AmyBa (Blue) and soybean β-amylases (PDB ID: 1Q6C) (gray) and D (PDB ID: 1Q6C) (gray). The C-terminal SBD in microbial β-amylases (box, purple) and the C-terminal loop in plants (box, red). Duan, X, et al., ibid.
The AmyBa has an additional starch binding domain at the carboxy terminus (Panel B) compared to soybean β-amylases (panel D).
Since no structures of (AmyBa are publically available, we present Figure \(\PageIndex{9}\), which shows an interactive iCn3D model of beta-amylase from Bacillus cereus var. mycoides in complex with maltose (1J0Z)
Figure \(\PageIndex{9}\): Beta-amylase from Bacillus cereus var. mycoides in complex with maltose (1J0Z). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...S2aWbL3z3jPnW8
Similar to alpha-amylase, β-amylase has an N-terminal catalytic domain with a beta-barrel, a connecting second domain, and a third C-terminal domain which is primarily antiparallel β-sheets. Two key catalytic side chains, Glu 172 and Glu 367, are found in the beta-barrel.
In Chapter 20.6, we discussed starch synthesis (not its hydrolysis) in detail. We showed that the reaction, which uses a NDP sugar as a glycan donor, could proceed either with retention or inversion of the anomeric carbon of the donor NDP-sugar. This is illustrated for the reaction of a C1 α-NDP donor monosaccharide with a monosaccharide acceptor to produce the α(1,4) link with retention of configuration or the β(1,4) link with inversion as shown in Figure \(\PageIndex{10}\) below.
Figure \(\PageIndex{10}\): Reaction of a donor NDP-monosaccharide and an acceptor monosaccharide with retention or inversion of configuration at the anomeric carbon of the donor
The same stereochemical outcomes are possible for the hydrolysis of acetal bonds by glycosyl hydrolases. Alpha-amylases cleave the α (1,4) glycosidic bonds to produce shorter dextrins (containing branched glucose units not cleaved by alpha-amylases), and α (1,4) linked glucose oligosaccharides of lengths from 2 glucose units (maltose) up to 7-8. This reaction hence proceeds with the retention of configuration. In contrast, beta-amylases cleave starch to produce maltose with an inversion of configuration at the anomeric-reducing end of the maltose. We explore the chemistry of retention and inversion more in the next section on cellulase, which cleaves the β (1,4) acetal link in cellulose, but in general, reactions that proceed with inversion react in an SN2 response, similar to the nucleophilic attack on alkyl halides. For the glycosyl transferases that proceed with inversion, the attacking nucleophile on the acceptor is made more nucleophilic by general base catalysis by a deprotonated glutamic or aspartic acid.
Figure \(\PageIndex{11}\) shows the results of in silicon docking studies of a small glycan, maltotetraose, to AmyBa.
Figure \(\PageIndex{11}\): Molecular docking of AmyBa with maltotetraose. The overall structure and substrate binding pocket analysis of AmyBa are shown.
AmyBa displayed very high amylase activity compared to other microbial β-amylases, and its enzymatic activity was much closer to sweet potato β-amylase. Molecular dynamic and docking programs can be used to calculate binding energies for substrates. The binding energy and enzymatic activities for bacteria and sweet potato β-amylase were highly correlated, suggesting that the extensive interactions of AmyBa and maltotetraose help drive catalysis by using the energy released on binding to lower activation energies for the reaction.
Saccharification
To enter glycolysis and fermentation, maltose must be converted to the monosaccharide glucose. The conversion of a polysaccharide to its monomers is called saccharification. To complete the conversion of starch to glucose, another enzyme, glucoamylase (also called amyloglucosidase and γ-amylase), is added. It is an exoglucosidase that cleaves both α (1,4) in amylose, amylopectin and maltose and α (1,6) branches, to form free glucose. It is a member of the glycoside hydrolase family 15 in fungi, glycoside hydrolase family 31 of human maltase-glucoamylase, and glycoside hydrolase family 97 of bacterial forms.
Fermentation
Glucose (C6H12O6) can now enter the glycolytic pathway and continue to ethanol after conversion of pyruvate to acetaldehyde by pyruvate decarboxylase and final conversion of acetaldehyde to ethanol by alcohol dehydrogenase:
C6H12O6 (s) → 2 CH3CH2OH (l) + 2CO2 (g) (anaerobic ethanol biosynthesis)
The yeast Saccharomyces cerevisiae catalyzes this entire pathway.
The final fermentation process yields a 12-15% ethanol solution, which is distilled to form a 95% ethanol/5% water azeotrope. The water is removed by adding zeolites (molecular sieves) which can adsorb water but not ethanol.
Life Cycle Analysis of Bioethanol: Is it better than fossil fuels?
We reiterate the promise of bioethanol to address global warming and climate change by presenting again the chemical equations that suggest that its use as a fuel is carbon neutral:
6CO2 (g) + 6H2O (l) → C6H12O6 (s) + 6O2 (photosynthesis)
C6H12O6 (s) → 2 CH3CH2OH (l) + 2CO2 (g) (anaerobic ethanol biosynthesis)
2CH3CH2OH (l) + 6O2 → 4CO2 (g) + 6H2O (g) (combustion of ethanol)
If only these three equations, this simple model for production and use of corn bioethanol, were the only factors influencing net CO2 emission on bioethanol burning, there would be no controversy about its use. Yet the actual CO2 emissions depend on many more hidden from view by these simple equations. What is needed is a life cycle analysis (LCA) that determines the environmental impact (in this case, net CO2 emissions) of corn ethanol through every phase of its existence, from cradle to grave, starting with the planting of corn to the combustion of bioethanol for transportation.
All models must be tested. It's easiest to start with the simplest model. If the data fit the model, great, you're done. If not, new, more expansive models must be developed and tested. Those vociferously supporting bioethanol use often use the simple stoichiometry evident in the three equations to state that bioethanol is carbon neutral. Most, however, would want a detailed life cycle analysis (LCA) before jumping to an immediate conclusion.
LCAs are very challenging, and data on a global scale is required. Some measurements at the worldwide scale have significant uncertainties (that don't include CO2 in the atmosphere, however) and are estimates, at best. A recent study looked at the impact of a specific event, the adoption of the US Renewable Fuel Standards (RFS) that regulate biofuels in the US (which produces about half of all the world's biofuels), on CO2 emission from the significant increase in corn plant and corn ethanol the followed the adoption of the standard. . Using LCA based on a series of economic and environmental metrics, the model shows that bioethanol is not a panacea for CO2 emissions and may be more detrimental than fossil fuels use for vehicles.
The study calculated the carbon intensity changes for corn ethanol that followed after the adoption of the standards. Scientists have used other events that led to immediate changes (9/11) and 1-2 year changes (Covid pandemic) on environmental parameters like CO2 emissions.
Carbon intensity measures how much energy-related CO2 is emitted per dollar generated (GDP). Ideally, policies should be implemented that decrease carbon intensity. Green energy derived from both solar and wind is an example. A similar metric is energy intensity, the total energy production per GDP, and both are consumption-based values.
Figure \(\PageIndex{12}\) shows carbon intensity per GDP per country over the last 30 years (data from Our World in Data).
Figure \(\PageIndex{12}\): Consumption-based carbon intensity from 1990 to 2018. Our World in Data.
Generally, the world is moving to more efficient energy production, but remember that our energy consumption is still dramatically increasing.
The LCA model showed that the RFS led to these interrelated outcomes. It:
- increased corn prices by 30% and the prices of other crops by 20%
- increased US corn cultivation by 2.8 Mha (8.7%) and total cropland by 2.1 Mha (2.4%) in the years following policy enactment (2008 to 2016). (1 hectare is an area of a square with100 meters sides, equivalent to 10,000 m2
- increased annual nationwide fertilizer use by 3 to 8%
- increased water quality degradation by 3 to 5%
- increased emissions from domestic land use changes
These all combined to lead to a carbon intensity of corn ethanol that was "no less than gasoline and likely at least 24% higher", according to the study.
The changes in the metric are visually described in Figure \(\PageIndex{13}\).
Figure \(\PageIndex{13}\): Changes due to the RFS. (A) Corn planted area. (B) Cropland area. (C) Carbon emissions. (D) Nitrogen applications. (E) Nitrous oxide emissions. (F) Nitrate leaching. (G) Phosphorus applications. (H) Soil erosion. (I) Phosphorus runoff. Positive numbers indicate an increase due to the RFS. Field-level results were aggregated at the county level for enumeration and visualization. Tyler J. Lark et al. PNAS. 119, 2022 (https://doi.org/10.1073/pnas.2101084119) Creative Commons Attribution License 4.0 (CC BY).
Land use changes include farming land that was retired or designated for conservation programs. Tilling additional land releases carbon stored in the soil. The increased farming significantly increased fertilizer production, which leads to N2O emissions. In addition, more of the existing cropland was planted with corn. These finds contrast with a USDA study that shows that corn ethanol has a 39% lower corn ethanol intensity than gasoline which was stated to derive from carbon captures from the newly planted crops. However, that study did not account for emissions from land use changes.
LCA can identify aspects of production that lead to the most negative consequences, which for the sake of this chapter is greenhouse gas emissions. For example, the LCA for corn ethanol might improve if the CO2 released on its production during anaerobic ethanol biosynthesis could be captured. Outcomes would also change if renewable energy sources were used for stages of production that require fossil fuel use.
This rigorous LCA did not address the moral question of using land that could be used to feed people to produce bioethanol for use in our ever-bigger vehicles. In addition, opponents of solar energy installations suggest that solar installs would require so much land that it would remove land for agricultural purposes. What is missing from their argument is the vast amount of land used now for corn ethanol. Farmers planted 90 million acres of corn in 2022 in the US, a land area about 90% the size of the entire state of California. 44% of that corn went to biofuels, and only 12% went to human consumption. In addition, approximately 44% percent was used to feed animals for human consumption, an inefficient and unsustainable use of crop land and resources.
Production of sucrose and bioethanol from sugarcane
Like corn, sugar cane, a tropical, perennial grass, is used (mainly in Brazil) to produce ethanol. Sugar cane is a C4 plant with a high ability to fix carbon. The fact that it is a perennial and does not need replanting each year makes it a more ideal feedstock than corn for bioethanol production. In 2020, sugar cane was by far the most-produced crop or livestock product in the world (1.87 billion metric tons), followed by corn (1.16 billion metric tons). The production by country for both corn and sugar cane is shown in Figure \(\PageIndex{14}\).
Figure \(\PageIndex{14}\): Corn and sugar cane production by country. Graphs from Our World in Data. https://ourworldindata.org/agricultural-production#
That sugar cane production is so high compared to grain crops that provide nutrition (not just "sweet" calories) might come as a surprise, but it shouldn't, given our addiction to sweet foods.
Sugar cane is often harvested manually in developing countries. It is then cut, milled, and mixed with water to extract the soluble sucrose (table sugar). The sugar cane components during extraction are shown below in Figure \(\PageIndex{15}\).
Figure \(\PageIndex{15}\): Components of Sugar Cane (after Larissa Canilha et al. J Biomed Biotechnol. 2012; 2012: 989572. doi: 10.1155/2012/989572
Sucrose is a nonreducing disaccharide (O-α-D-glucopyranosyl-(1,2)-β-D-fructofuranoside). Its structure is shown in Figure \(\PageIndex{16}\).
Figure \(\PageIndex{16}\): Structure of fructose
Sucrose decomposes at 186 °C (367 °F) instead of melting (a feared event for organic chemistry students who wish to record melting temperatures in the lab) to form caramel. Molasses is a very viscous liquid product from refining sugar cane or sugar beets. It is used as a sweetener with its own taste properties, and it's a component of brown sugar as well. On a sweetness scale, if sucrose is assigned a value of 100, fructose is 140, high fructose corn syrup is 120-160, and glucose is 70-80.
For bioethanol production, sucrose is degraded by the enzyme invertase to form monomeric glucose and fructose. Invertases are activated on the milling and liquification of the sugar cane, so if sucrose is the desired commercial product, an additional clarification step (heat to 115°C and treat with lime and sulfuric acid) is necessary to prevent hydrolytic cleavage of sucrose.
Bioethanol production from sugar cane sucrose
Bioethanol can be made from either the fibrous lignocellulose remains of the sugar cane, called bagasse or from water-soluble sucrose. We will describe the production of cellulosic ethanol from field crop stalks, called stover, and leaves, straw, wood chips, and sawdust (all "waste" biomass), in Chapter 31.5. The same principles apply to bioethanol production from bagasse, the solid remains after the juice extraction from sugar cane. (Bagasse is often burned to provide energy for sugar cane processing).
In addition to sugar cane, sugar beets and sweet sorghum, a C4 plant similar to sugar cane, are used to produce bioethanol. As a C4 plant, sweet sorghum is very efficient at producing biomass through photosynthesis. It grows in temperate and tropical climates, has a short growing period, and is resistant to drought and cold. Its stalks have free sugars as well as lignocellulose stocks.
This chapter will focus on bioethanol production from sugar cane sucrose. Again, this is accomplished using yeast (Saccharomyces cerevisiae), which has the enzyme invertase 2 (beta-fructofuranosidase 2 or Saccharase) needed to convert sucrose into sucrose fructose and glucose, which can enter glycolytic and fermentation pathways.
Invertase, shown in 1842 to invert the stereochemistry of sugars, was first isolated from yeast in 1860. It has a secreted glycosylated homooctameric form and an intracellular form, all products of the same gene. It's a member of Family 32 of the glycoside hydrolases. The structure of the Saccharomyces invertase (SInv) octamer structure is shown in Figure \(\PageIndex{17}\) below.
Figure \(\PageIndex{17}\): Structure of octameric SInv. M.Angela Sainz-Polo et al. JBC, 288, 9755-9766 (2013). DOI:https://doi.org/10.1074/jbc.M112.446435. Creative Commons Attribution (CC BY 4.0)
Panel a shows a view of the SInv octamer in ribbon (left) and solvent-accessible surface (right) representations, showing each subunit in a different color.
Panel b shows that the octamer is rotated 90°, illustrating that it can be best described as a tetramer of two different kinds of dimers, AB/CD and EF/HG, which are compared by superimposing subunit F on subunit B in c
Even though all eight subunits in the octamer are identical (58.5K, 512 aa), the quaternary structure of the 8-mer can best be viewed as a tetramer of dimers (i.e. 4 dimers pack to form two packed tetramers giving the octamer). The AB and CD dimers pack in a "closed form" with a narrow active site pocket, allowing a glycan of 3-4 monomers. The EF and GH dimers pack in an "open form" with a wide active site pocket for longer glycans. Of course, our main interest here is in the binding of sucrose.
Figure \(\PageIndex{18}\) shows an interactive iCn3D model of Saccharomyces cerevisiae invertase (4EQV)
Figure \(\PageIndex{18}\): Saccharomyces cerevisiae invertase (4EQV). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...ysQW8YSrvPkus9
The color coding of the subunits is the same as shown in the right top image of Panel A, Figure 16 above.
The GH32 enzymes, including invertase, have a catalytic domain consisting of a 5-bladed β-propeller, with each blade having four antiparallel beta-strands. The blades surround an active site enriched in carboxyl side chains.
The "closed" form active site of the AB and CD dimers has at its base Phe 388 and Phe 296, which provide hydrophobic interactions. The "open" form active site of the EF and GH dimers also has a salt bridge between Asp 45 and Lys 385. These are shown in Figure \(\PageIndex{19}\), along with a bound 1-kestose, which is a trisaccharide"sucrose analog" found in vegetables. It consists of a β-D-fructofuranose connected to β-D-fructofuranosyl and α-D-glucopyranosyl residue at the 1- and 2-positions.
Figure \(\PageIndex{19}\): Dimer interface at the active site. The octameric SInv active site interfaces are detailed, keeping the same color pattern as above with one subunit being shown in ribbon representation for clarity. Angela Sainz-Polo et al. JBC, 288, 9755-9766 (2013). DOI:https://doi.org/10.1074/jbc.M112.446435. Creative Commons Attribution (CC BY 4.0)
Panel A shows that the AB/CD dimers are tightly made by interactions among both their catalytic and β-sandwich domains. Hydrophobic interactions around found at the base of the catalytic pocket through Phe-388 and Phe-296.
Panel b, by contrast, shows that the EF/GH dimers interact only through their β-sandwich domains. In addition, the catalytic pocket is also paved by a new salt bridge formed between Asp-45 and Lys-385 from the β-sandwich domain, which lines the cavity. A putative 1-kestose molecule is shown in a spherical representation.
The hydrolysis of sucrose by invertase proceeds with the retention of configuration at the anomeric carbon. An active site Aspartate 22 acts as a nucleophile to form a glycosylated intermediate (fructose-Asp). This is followed by hydrolysis of the intermediate. An active site Glutamate 203 acts as a general acid/base. The fructose could also be transferred to another glycan in a transglycosylation reaction. The hydrophobic side chains Phe-388 and Phe-296 line the base of the active site pocket.
Figure \(\PageIndex{20}\) shows an interactive iCn3D model of the AB dimer of Saccharomyces cerevisiae invertase with key active site residues (4EQV)
Figure \(\PageIndex{20}\): AB dimer of Saccharomyces cerevisiae invertase with key active site residues (4EQV). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...hX9FbxLLip1f97
Figure \(\PageIndex{21}\) shows an interactive iCn3D model of the EF dimer of Saccharomyces cerevisiae invertase with key active site residues (4EQV). It has an additional salt bridge between Asp-45 and Lys-385.
Figure \(\PageIndex{21}\): EF dimer of Saccharomyces cerevisiae invertase with key active site residues (4EQV). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...Dh33BtT5c8GXK6
Life Cycle Analysis of Sugar Cane Ethanol
Does the production of bioethanol from sugar cane lead to lower net CO2 emissions than bioethanol produced from corn? The answer would depend on if sucrose (first generation) or lignocellulose (second generation) from bagasse is the feedstock.
A recent LCA has been performed on the first-generation (feedstock is sucrose) production of bioethanol from sugar cane in Ecuador. There is a lower cost of production from this sugar-based feedstock, which requires just grinding and the addition of yeast for fermentation. It does not require a saccharification step.
Figure \(\PageIndex{22}\) shows the various stages and processes used to perform LCA on the bioethanol production from sugar cane sucrose. It's presented to show the complexity of such analyses, so look at the detail only if you are especially interested.
Figure \(\PageIndex{22}\): Anhydrous ethanol life cycle system boundaries and main product flows quantification for year 2018. Arcentales-Bastidas, D.; Silva, C.; Ramirez, A.D. The Environmental Profile of Ethanol Derived from Sugarcane in Ecuador: A Life Cycle Assessment Including the Effect of Cogeneration of Electricity in a Sugar Industrial Complex. Energies 2022, 15, 5421. https://doi.org/10.3390/en15155421. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
The study analyzed four stages for analysis, agricultural, milling, distillation and electricity generation (presumably by burning the byproduct bagasse) for impacts. They defined two functional units:
- 1 ton of sugarcane "at the farm gate” for the agricultural stage;
- 1 L of ethanol "at the plant (factory) gate”.
The key results are as follows:
- The global warming potential (GWP) impact at the farm gate level was 53.6 kg of carbon dioxide equivalent (kg CO2 equiv) per ton of sugarcane produced; This arose mostly from N2O (34%), a potent greenhouse gas released in the process, and diesel fuel used in agricultural machinery (24%).
- The GWP for 1 L of ethanol produced at the plant gate was 0.60 kg CO2 equiv, with the distillation phase contributing the most.
Before proceeding further, let's explain the key term, global warming potential (GWP), which is widely used in LCA. It adds the contribution of other greenhouse gases like methane (CH4) and nitrous oxide (N2O), each of which has unique IR absorption spectra and atmospheric half-lives. The IPCC uses a 100-year time frame for the calculation of the GWP, and uses this formula:
CO2 Equivalent kg = CO2 kg + (CH4 kg x 28) + (N2O kg x 265)
\begin{equation}
\mathrm{CO}_2 \text { equivalent } \mathrm{kg}=\mathrm{CO}_2 \mathrm{~kg}+\left(\mathrm{CH}_4 \mathrm{~kg} \times 28\right)+\left(\mathrm{N}_2 \mathrm{O} ~k g \times 265\right)
\end{equation}
- CO2 has GWP of 1 by definition since it is the reference. Its time frame in the atmosphere (100s to 1000 years) doesn't matter since it is the reference.
- CH4 has a GWP of around 27-30 over 100 years. It reflects its higher IR absorbance but lower lifetime (around 12 years).
- N2O has a GWP of around 265-273 over a 100-year timescale. N2O has a lifetime of around 109 years.
The equation can be amended by adding other greenhouse gases released in manufacturing and the use of refrigerants. These include Freon-12 (Dichlorodifluoromethane) (CFC-12, with a lifetime of 100 years and a GWP100 of 10,200, and SF6 (used in the electricity industry to keep networks running safely and reliably) with a lifetime of 3200 years and GWP00 of 23,500!
N2O (laughing gas) is an overlooked source of greenhouse gases, but it leads to about 7% of the warming effect of the greenhouse gases with long life-times and a high GWP100. Agricultural practices lead to about 65% of its total emission. It is a component of the soil and atmosphere nitrogen cycle. In soil, its concentration depends on soil microbes that engage in nitrification and denitrification processes. These in turn depend on the amount of fixed nitrogen, oxygen levels and metabolically available carbon sources. The nitrification reaction, which occurs in aerated and moist soils, involves the oxidation of NH3↔NH4+ to NO2 and NO3-, with some N2O release. The major source of N2O occurs under anaerobic conditions. These general reactions are shown below.
- Nitrification (aerobic, oxidation): N2 → (NH3 ↔ NH4+) → NO2 → NO3-
- Denitrification (anaerobic, reduction): NO3− → NO2− → NO → N2O → N2
In anaerobic sites, NO is an electron receptor during microbial respiration. N2O is produced when there is excess nitrogen available (past the needs of plants and microorganisms), so excess use of fertilizers and manure increases its production. Nitrifying and denitrifying bacteria are most active in producing N2O in environments with abundant N relative to assimilatory demands by other microorganisms or plants (Firestone and Davidson, 1989), as is often the case following soil amendment of fertilizers, manure, or crop residues. Physical processing of the soil (such as tillage) also affects N2O emissions by introducing crop residues in the soil, changing soil particle size and surface area, and by changing the porosity of the soil. All of these affect soil substrate/product availability and their aqueous and gas diffusion rates.
Let's use dimensional analysis from introductory chemistry to convert the GWP from the farm gate/agricultural stage (53.6 kg CO2 equiv/ton of sugar cane) to kg CO2 equiv/1L of ethanol (EtOH) so we can add it the GWP from the pant gate, which is expressed in kg CO2 equiv/L ethanol produced. The dimensional conversion is shown in Table \(\PageIndex{2}\) below.
| 53.6 kg CO2 equiv | 1 ton SC | 1 L Juice | = | 0.1 kg CO2 equiv |
| 1 ton SC | 800 L juice | 0.7 L EtOH | 1 L EtOH produced |
Table \(\PageIndex{2}\): Conversion of 53.6 Kg CO2 equiv/ton of sugar cane from the farm gate (left hand column) to 0.1 Kg CO2 equiv/1L of ethanol (EtOH).
Now add this to the reported 0.60 kg CO2 equiv/1 L of ethanol from the plant (factor) gate and you get a total of 0.7 kg CO2 equiv /1 L ethanol produced.
Now use dimensional analysis from introductory chemistry to calculate how much CO2 is actually produced on the combustion of ethanol. That value is calculated in Table \(\PageIndex{3}\) below.
| 1 L EtOH | 1000 mL EtOH | 0.789 g EtOH | 1 mol EtOH | 4 mol CO2 | 44 g CO2 | 1 kg CO2 | = | 1.5 kg CO2 |
| 1L EtOH | 1 mL EtOH | 46 g EtOH | 2 mol EtOH | 1 mol CO2 | 1000 g CO2 | 1 L EtOH |
Table \(\PageIndex{3}\): Total Kg CO2 produced on combustion of 1 L of ethanol (EtOH)
The promise of bioethanol is that for every 1 C atom used to create it, 1 C atom would be released. We saw that the LCA analysis for corn ethanol in the US did not meet that expectation. In the Ecuadorian analysis, it appears that it did, since 0.7 kg CO2 equivalents is required to produce 1 L of bioethanol from sugar cane, but 1.5 Kg CO2 is released on its burning.
The LCA analysis described above reflects just the global warming potential for the use of sugar cane sucrose for bioethanol production. However, bioethanol production from sugar cane juice has other negative impacts as listed in Table \(\PageIndex{4}\) below.
| Impact Category | Characterization Factor | Reference Unit |
|---|---|---|
| Climate change | Climate change—GWP100 | kg CO2eq. |
| Freshwater eutrophication | Freshwater eutrophication potential—FEP | kg Peq. |
| Marine eutrophication | Marine eutrophication potential—MEP | kg Neq. |
| Abiotic depletion | Metal depletion—MDP | kg Feeq. |
| Photo oxidant formation | Photochemical oxidant formation potential—POFP | kg NMVOCeq. |
| Particulate matter emissions | Particulate matter formation potential—PMFP | kg PM10eq. |
| Terrestrial acidification | Terrestrial acidification potential—TAP100 | kg SO2eq. |
- Biofuels are renewable energy sources derived from biomass, such as plant materials and waste.
- Corn and sugar cane ethanol are two examples of biofuels that are produced by fermenting the sugars found in these crops.
- Corn ethanol is typically produced by converting the starch in corn kernels into glucose, which is then fermented to produce ethanol.
- Sugar cane ethanol is produced by crushing the cane to extract the juice, which is then fermented to produce ethanol.
- Both corn and sugar cane ethanol are primarily used as a gasoline additive to increase octane and reduce emissions.
- Corn ethanol is a controversial biofuel because of the high amount of energy used to produce it and the impact on food prices and the environment.
- Sugar cane ethanol is considered to be a more sustainable biofuel because it is less energy-intensive to produce and can be produced on land not suitable for food crops.
- However, large scale production of sugar cane ethanol has also been criticized for leading to deforestation, loss of biodiversity and displacement of local communities.
- Cellulosic ethanol, made from non-food feedstocks like switchgrass or wood chips, is considered to be a more sustainable biofuel alternative to corn and sugar cane ethanol.



.png?revision=1&size=bestfit&width=541&height=341)
.png?revision=1&size=bestfit&width=369&height=336)
.png?revision=1&size=bestfit&width=374&height=373)
.png?revision=1&size=bestfit&width=571&height=374)
.png?revision=1&size=bestfit&width=593&height=395)