32.3: Climate Change - The Carbon Cycle and Carbon Chemistry
- Page ID
- 98019
Search Fundamentals of Biochemistry
The Carbon Cycle
In the last chapter section, we used oxygen isotopes in ice and ocean sediment cores going back millions of years ago to address the history and mechanisms of climate change. We focused on 18O/16O ratios in H2O and in calcite shells (CaCO3 ), and their corresponding δ18O values in ice and sediment cores, to determine CO2 and temperature over climatic history. Now it's time to talk about the other key atom, C, the ratio of 13C/12C and corresponding δ13C values, not only in CaCO3 but also in CO2 and the organic molecules it transforms into through photosynthesis and the heterotrophic organisms that consume them. 13C partitions not only into inorganic carbon but also into organic molecules throughout life. Hence we need a more detailed understanding of the carbon cycle.
The carbon cycle is likely discussed in introductory chapters in biology textbooks, but probably never in chemistry texts. It is fundamental to an understanding of climate, its control and change, and human processes that alter it. Figure \(\PageIndex{1}\) shows a representation of the carbon cycle. Calculated amounts of carbon found in the lithosphere (the solid part of the earth), the atmosphere (specifically the lower part, the troposphere), and the hydrosphere are shown. (The cryosphere, the frozen ice found in Greenland and Antarctica, is not shown). The biosphere includes part of each of these "spheres" that harbor life. Since life has been shown to exist 10 km down in the crust, we'll refer to the entire region in the diagrams as the biosphere. Figure 1 presents carbon stores in petagrams (1015 g) or gigatons of carbon (GtC), as 1 petagram equals 1 billion metric tons (or approximately 1.1 billion US tons).
Figure \(\PageIndex{1}\): The carbon cycle. https://commons.wikimedia.org/wiki/F...te_diagram.svg
In addition to the total amount of carbon stored in each region, (GtC), the net changes in carbon per year as it moves into and out of reserves (GtC/yr) are shown in blue arrows with attached numbers.
The exchanges of carbon in the cycle occur at different time scales. Geologically "fast" exchange, on a time scale up to 1000s of years, occurs among the oceans, atmosphere, and land, while a slow exchange (over hundreds of thousands to millions of years) occurs in deep soils, deep ocean sediments, and rocks. We will mostly consider exchanges among the atmosphere, land, and oceans.
CO2 in the terrestrial biosphere is removed by photosynthesis and returned by respiration by autotrophs like plants, and heterotrophs like microbes that consume soil carbon and plant remains. CO2 in the atmosphere is also removed by ocean autotrophs like ocean phytoplankton and through partitioning into dissolved inorganic carbons (DIC) molecules like HCO3- and CO32- into the oceans.
Before we probe some relevant reactions within it, let's look at the big picture and perhaps the most relevant to our climate crisis - the factors causing our increasing CO2 atm and global warming. To do that, we must put numbers on the cycle to quantify it.
Quantitating the carbon cycle
In Chapter 31.1, we used parts per million (ppm) as a unit for expressing the amount of CO2 in the atmosphere. Table \(\PageIndex{1}\) below shows how to translate the percentage (parts per 100) for each component gas in the atmosphere (with which you are familiar) into ppm.
| Gas | % (parts per 100) in atm | part per million |
|---|---|---|
| N2 | 78.09 | 780,900 |
| O2 | 20.94 | 209,400 |
| Ar | 0.93 | 9300 |
| CO2 | 0.0415 | 415 |
Table \(\PageIndex{1}\): Unit conversion - % to ppm
Climate scientists use ppm instead of concentration (in molecules/m3) since they wish to know the relative percent or ppm increase with time, which does not depend on temperature and pressure. In contrast, concentration does depend on T and P, as you will remember from the ideal gas law, PV=nRT or n/V=P/RT that you studied in introductory chemistry.
It is important to use dimensional analyses to interconvert units as well. Table \(\PageIndex{2}\) below shows conversion factors to switch between GtC, Gt CO2, and ppm.
| Convert from | to | conversion factor |
|---|---|---|
| GtC (Gigatons of carbon) | ppm CO2 | divide by 2.124 |
| GtC (Gigatons of carbon) | PgC (Petgrams of carbon) | multiply by 1 |
| Gt CO2 (Gigatons of carbon) | GtC (Gigatons of C) | divide by 3.664 = 44.01/12.01) |
| GtC (Gigatons of carbon) | MtC | multiply by 10000 |
Table \(\PageIndex{2}\): Unit conversion - GtC and Gt CO2
To be more technical, atmospheric CO2 concentrations are expressed in mol fraction of CO2 in the dry air atmosphere. The ppm for CO2 is hence μmol CO2 per mole of dry air.
We have to put numbers on the components of the carbon cycle to quantitatively analyze changes in its components, otherwise, we can't know what is presently happening nor will we be able to predict with some certainty the future. Stoichiometry and reaction kinetics are probably the least liked parts of chemistry for many, but they are critical in understanding climate change We have to apply them on a global scale. Two key terms are important:
Stocks or reserves: How much carbon (mass in Gigatons or petagrams) is stored in given locations in the biosphere. This allows us to understand what % of all carbon stocks are in the ocean, for example. Stocks are usually reported as gigatons of carbon (GtC), not gigatons of carbon dioxide, since many stocks (like fossil fuels) consist of mostly C and H without oxygen. As in stoichiometry calculations in introductory chemistry courses, GtC in the atmosphere can be converted to gigatons of CO2 by using dimensional analysis.
Fluxes (rates): How much carbon is transferred from one reserve to another per year (Gigatons/yr). Climate scientists are simply applying the Law of Mass Conservation that you learned in introductory chemistry, to the entire biosphere.
Figure \(\PageIndex{2}\) shows the reserves/stock (GtC) for reserves and decadal (2012-2021) average fluxes (large and small arrows, GtC/yr) for individual or aggregated stocks.
Figure \(\PageIndex{2}\): Schematic representation of the overall perturbation of the global carbon cycle caused by anthropogenic activities averaged globally for the decade 2012–2021. E represents emission and S "sink". Earth Syst. Sci. Data, 14, 4811–4900, 2022. https://doi.org/10.5194/essd-14-4811-2022. © Author(s) 2022. This work is distributed under the Creative Commons Attribution 4.0 License.
The abbreviations used are: EFOS (emissions, fossil fuels), ELUC (emissions land use changes - mostly deforestation), SLAND (terrestrial CO2 sink), SOCEAN (ocean CO2 sink), GATM (Growth Rate CO2 atm), BIM (carbon budget imbalance). Uncertainties are also shown except for the atmospheric CO2 growth rate which is known precisely and accurately through modern measurements. (It's also the easiest to measure). Human (anthropogenic changes) occurs on top of the carbon cycle.
The upward arrows indicate release into the atmosphere and the downward arrows the absorption in the oceans and land. The thickness of the arrows gives a relative measure of the size of emission or absorption. The thickest arrow and highest value (9.6 GtC/yr) is for the anthropogenic emission of carbon from our use of fossil fuels. Think about that! Humans are presently the biggest contributor to the carbon cycle. Before the industrial revolution, human contributions were minimal.
Figure x above shows that on average, 9.6 GtC/yr was released from fossil fuel use between 2012-2021. The actual flux of anthropogenic carbon release in 2021 was 9.9 GtC, equivalent to 36.4 Gt CO2.
If you add the up arrows and subtract from that sum the down ones, you get +4.8 GtC/yr. This represents the net average increase in GtC in atmosphere CO2 per year for 2012-2021. That is close to the accurately, and precisely known value of +5.2 GtC/yr increase from the CO2 we pour into the atmosphere through our use of fossil fuels. Hence the figure above is a bit out of balance (about 0.3 GtC too low - the BIM carbon budget imbalance), but given the difficulty in calculating these values, it is remarkably close to "mass balance" as you learned in introductory chemistry classes. In general, before the industrial revolution, the sum of the fluxes leading to the addition of CO2 into the atmosphere was equal to the sum of the fluxes that removed it. That is, the system was in a steady state. That is no longer the case.
Figure \(\PageIndex{3}\) shows a breakdown of the factors contributing to annual (left) and cumulative (right) fluxes of carbon (GtC/yr), a metric for CO2 flux, over time since 1850.
Figure \(\PageIndex{3}\): Combined components of the global carbon budget as a function of time for fossil CO2 emissions (EFOS, including a small sink from cement carbonation; grey) and emissions from land-use change (ELUC; brown), as well as their partitioning among the atmosphere (GATM; cyan), ocean (SOCEAN; blue), and land (SLAND; green). Panel (a) shows annual estimates of each flux ( GtC yr−1, and panel (b) shows the cumulative flux (the sum of all prior annual fluxes) since the year 1850. Again, the graph shows GtC not Gt CO2. . © Author(s), ibid
You might ask why the atmospheric growth in CO2 (shown in green) is negative. We'll answer that question below.
Lastly, let's think about the total cumulative changes in GtC released and absorbed since 1850 (pre-US civil war and before the big release of CO2 in modern times). Those data are shown in a bar graph in panel A of Figure \(\PageIndex{4}\). The bar graph in the right panel shows the mean decadal averages that are shown in Figure 2 above.
Figure \(\PageIndex{4}\): Total cumulative changes in GtC released and absorbed since 1850 (panel A) and mean decadal fluxes (panel B). EFOS (emissions, fossil fuels), ELUC (emissions land use changes - mostly deforestation), SLAND (terrestrial CO2 sink), SOCEAN (ocean CO2 sink), GATM (Growth Rate CO2 atm), BIM (carbon budget imbalance). © Author(s), ibid
The positive emission and negative absorption contributions are easy to see in the bar graph. The blue bar represents the net emission of carbon from fossil fuels and fills the gap to complete mass balance as we discussed above. It also explains the negative blue region in Figure 3. Just keep in mind that the blue net flux from fossil fuels is positive.
The cumulative contributions from fossil fuel emissions required to close the gap and fulfill mass balance is is +275 GtC, which when multiplied by the conversion factor (1ppm/2.124 GtC) translates into a 129.5 ppm increase in atmospheric CO2 over that time. This is very close to independent measurements of a rise of 129.3 ppm (14.7-284.7) over that time.
The data from Figure \(\PageIndex{4}\) has been entered in the first four columns of Table \(\PageIndex{3}\) below.
| Source | Subtype | Stock reserves (GtC) |
J (Fluxes) GtC/yr + emission |
J=kapp[stock] |
|---|---|---|---|---|
| Atmosphere | - | 875 | ||
| Buried Fossil Fuels | Coal | 560 | +9.6 |
J=+9.6=k[905] (905 is sum of stocks) k=0.0106 |
| Oil | 230 | |||
| Gas | 115 | |||
| Terrestrial | Permafrost | 1,400 |
+1.2 (Land use Δ) |
Juse=+1.2=kuse[3550]; kuse=0.000338 |
| Soil | 1,700 | |||
| Vegetation | 450 | |||
| Oceans | Coasts | 10-45 | -2.9 |
J=-2.9=k[875] |
| Ocean Surface Sediments | 1,750 | |||
| Organic carbon | 700 | |||
| Marine Biota | 3 | |||
| Dissolve Inorganic Carbon (DIC) | 37,000 |
We can use this data to develop our own crude computational model predicting future CO2 emissions using Vcell, the program we used to produce time course (concentration vs time) graphs for both simple and coupled signal transduction reaction pathways.
Vcell can be used to calculate fluxes (J) in reaction pathways, where J is the change in concentrations of a species with time, given the initial concentration or amount of a reactant, and the rate constants affecting its production or removal. If we use the amount of carbon (GtC) in each reservoir in the biosphere and crust as a relative measure of "concentration" and the known fluxes (GtC/yr) for the transfer of carbon to and from the atmosphere as given in Table 3, we could calculate an "apparent rate constant for each flux using this equation:
Jstock = kapp[stock] (where stock is given in GtC).
\begin{equation}
\mathrm{J}_{\text {stock }}=\mathrm{k}_{\mathrm{app}}[\text { stock in } \mathrm{GtC}]
\end{equation}
These "apparent" rate constants are needed to run the Vcell simulation that can reproduce the actual fluxes shown in Figures 2 and 4). The simulation can be run over time
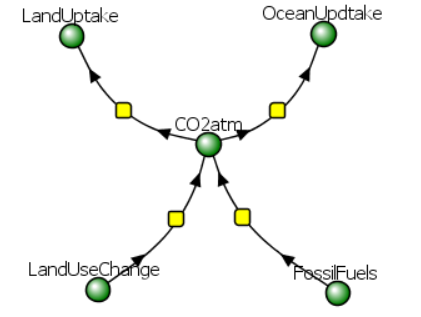
A simple four-term model based on Figures 2 and 4 is shown below. Run the simulation and see how atmospheric CO2 changes with time. This model is offered only to show how climate models are made and used, and also for fun. The graphs are valid and sound based on the input parameters, but the outputs are based on many assumptions that vastly simplify the model. For example, it assumes that no new input of fossil fuel from new drilling/mining occurs.
Alter the sliders on the model to change the rates of removal of CO2 atm through land uptake and ocean uptake. Also, change the rate of input of CO2 atm through land use changes and burning of fossil fuels. Try to "flatten" the curve earlier and to decrease CO2 atm faster.
You can download the spreadsheet and plot the individual contributions to CO2 atm as well.
This simulation shows CO2 atm levels peaking at about 982 GtC in 51 years (2073) from its average decadal (2011-2021) value of 875. That is an increase of 107 GtC over now (50 ppm CO2 rise from the present 414 to 463 ppm). Over 50 years, this gives an average annual rise of 2.14 GtC/yr or about 1 ppm CO2/yr. A comparison of the predicted atmospheric CO2 (ppm) levels through 2100 for the IPCC SSP1-2.6 scenario (blue) and simple Vcell model (red) is shown in Figure \(\PageIndex{5}\).
Figure \(\PageIndex{5}\): Predicted atmospheric CO2 (ppm) for SSP1-2.6 scenario (blue) and simple Vcell model (red)
SSP1-2.6 data - History: Meinshausen et al. GMD 2017 (https://doi.org/10.5194/gmd-10-2057-2017); Future: Meinshausen et al., GMD, 2020 (https://doi.org/10.5194/gmd-2019-222). https://climateanalytics.org/media/g...-3571-2020.pdf. https://gmd.copernicus.org/articles/13/3571/2020/
However imperfect the Vcell model is (incorrect assumptions, lack of complexity and feedback mechanisms, etc), the results shown above are veryclose to the projected increases in carbon dioxide in ppm described in IPCC reports for the SSP1-2.6 socioeconomic pathways, shown in the right panel (dark blue line) of Figure \(\PageIndex{6}\). This pathway predicts a rise of approximately 1.80 C in average global temperatures.
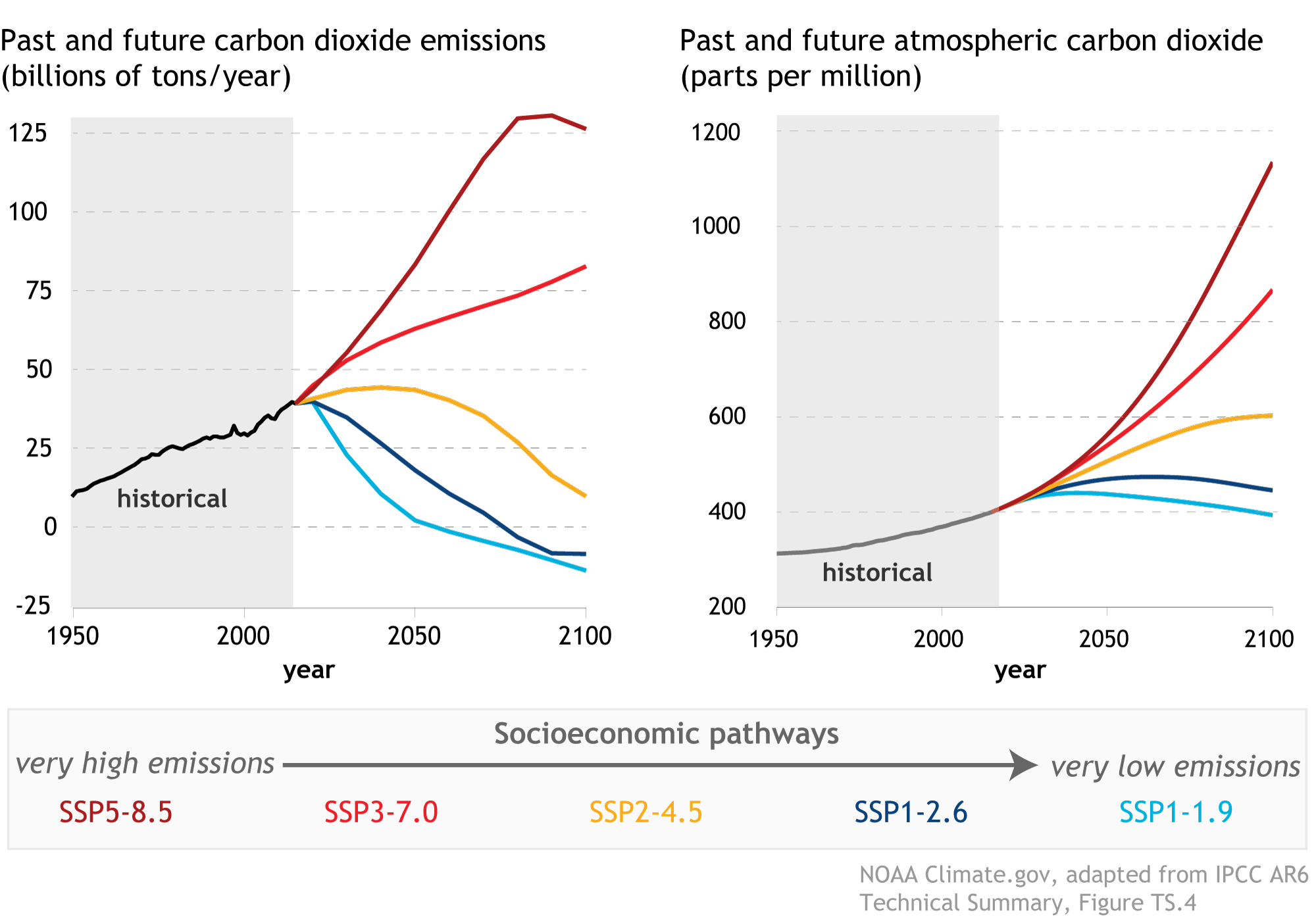
Figure \(\PageIndex{6}\): IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I
to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L.
Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K.
Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)].
Again, remember that the model is based on a ten-year average of CO2 emissions. Think of all the other assumptions in this model (other than the stock reserves and fluxes) that would give higher or lower values of future CO2 levels. One major one is that flux values are all held constant to allow calculations of the apparent rate constants for Vcell use. The model depletes much of the fossil fuel reserve. In addition, CO2 emissions in 2021 were actually 9.9 GtC/yr and going up!
In addition, a change in one parameter can affect the others. For example, the net uptake of atmospheric CO2 into the land and oceans has increased from 1960-2010, which makes sense given increased CO2 in the air forcing additional uptake (think LaChatelier's Principle). The oceans have taken up nearly 40% of the CO2 from fossil fuel use since the Industrial Revolution. If the rate of uptake decreases (i.e. if we start to saturate the uptake into oceans), CO2 accumulation in the atmosphere would accelerate. Data also suggest that if we successfully decrease CO2 in the atmosphere, the oceans would respond by decreasing uptake as well, which would slow the progress in reducing temperatures.
An interesting example relating atmospheric and ocean CO2 occurred from 1990-2000 when it has been shown that the ocean acted as a weaker sink. This occurred because of a decreasing gradient (the Δ or"effective concentration differences") between atmospheric CO2 and ocean "CO2", which decreased the ability of the ocean to act as a sink for CO2. You can decrease the Δ in two ways:
- decreasing the rate of entry of CO2 into the atmosphere from fossil fuel use. There indeed was a temporary slowdown in this decade.
- paradoxically, by briefly making the ocean in a shorter term a better sink. This happened in 1991 after the eruption of Mt. Pinatubo, which led to decreased air and ocean temperatures. CO2 is a nonpolar gas, which has higher solubility in water at lower temperatures (think about soda). This was a short-term and more minor effect than the decreased rate of fossil fuel emissions.
More complex models with more terms for emissions and absorptions of CO2 can be made. One is shown in Figure \(\PageIndex{7}\). This model adds CO2 release from the soil through respiration by microorganisms, as well as from plant respiration (CHO to CO2atm). Another term has been added for release by oceans.
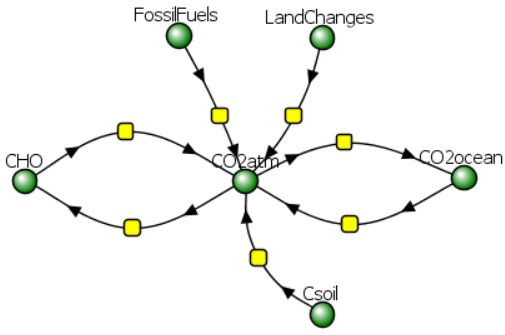
Figure \(\PageIndex{7}\): More complicated Vcell climate model.
Fortunately, we don't have to rely on these simple models to predict future trends in temperature and CO2. A complex dynamic model simulator that is in accord with many different climate models is available at your fingertips. Developed at MIT and Climate Interactive, and available for free from any web browser, the EN-ROADS program allows users to change sliders for key inputs and see future predicted temperature and CO2 levels. In accord with RCP and SSP IPCC pathways that tie future emissions to socio-economic policies (discussed in Chapter 31.1), the program allows users to change variables such as carbon pricing and incentives to move to clean energy in transportation, building and energy supplies sectors. Access the program directly from this page by clicking the Close icon in the program window in Figure \(\PageIndex{8}\) below.
Figure \(\PageIndex{8}\): EN-ROADS global climate simulator
Here is also an external link to the En-Roads global climate simulator (Developed by Climate Interactive, the MIT Sloan Sustainability Initiative, and Ventana Systems).
Move the interactive sliders and see the effect on greenhouse gas emissions and global temperatures. Here is a link to a one-page tutorial on its use.
At this climate meeting in Dubai, delegates agreed for "the need for deep, rapid and sustained reductions in greenhouse gas emissions in line with 1.5 °C pathways and calls on Parties to contribute to the following global efforts, in a nationally determined manner, taking into account the Paris Agreement and their different national circumstances, pathways and approaches". These included:
(a) Tripling renewable energy capacity globally and doubling the global average annual rate of energy efficiency improvements by 2030;
(b) Accelerating efforts towards the phase-down of unabated coal power;
(c) Accelerating efforts globally towards net zero emission energy systems, utilizing zero- and low-carbon fuels well before or by around mid-century;
(d) Transitioning away from fossil fuels in energy systems, in a just, orderly, and equitable manner, accelerating action in this critical decade, so as to achieve net zero by 2050 in keeping with the science;
(e) Accelerating zero- and low-emission technologies, including, inter alia, renewables, nuclear, abatement and removal technologies such as carbon capture and utilization and storage, particularly in hard-to-abate sectors, and low-carbon hydrogen production;
(f) Accelerating and substantially reducing non-carbon-dioxide emissions globally, including in particular methane emissions by 2030;
(g) Accelerating the reduction of emissions from road transport on a range of pathways, including through the development of infrastructure and rapid deployment of zero- and low-emission vehicles;
(h) Phasing out inefficient fossil fuel subsidies that do not address energy poverty or just transitions, as soon as possible.
Here is a link to an EN-ROADS climate model that shows the effects of the actions recommended at COP28. Explore the model by moving sliders and returning them to the preset positions. These models shown that aggressive action in all sectors that contribute to climate change would bring down projected temperature increases to 1.7 0C (3 0F), still above the originally recommended 1.5 0C limit. Of course this assumes that the world has the political will to carry them out.
The COP28 was held in a "petrostate" whose main revenue is from fossil fuels (30% of its GDP derives from them), so at the start of the meeting, it was unclear if any strides could be made to reduce fossil fuel production and use, the main cause of climate change. In fact, the fossil fuel industry has been (and likely still is) a main source of misinformation on climate change.
We should all be skeptical of models, especially ones that predict changes 80 or more years into the future. We gain confidence in a model if it accurately fits data going back in time and into the future data as well. We mentioned in Chapter 31.1 that oil company scientists knew of the likely climatic effects arising from fossil fuel emissions, but the company executives did not act on their models. Their models were startlingly accurate as shown in Figure \(\PageIndex{9}\) below, which shows their predictions for both CO2 levels and the associated increases in temperature caused by them.
Figure \(\PageIndex{9}\): Historically observed temperature change (red) and atmospheric carbon dioxide concentration (blue) over time, compared against global warming projections reported by ExxonMobil scientists. Supran, G., Rahmstorf, S., and Oreskes, N. Assessing ExxonMobil's global warming projections. Science (2023). https://www.science.org/doi/abs/10.1...cience.abk0063. Reprinted with permission from AAAS. Not for reuse.
Panel (A) shows “Proprietary” 1982 Exxon-modeled projections.
Panel (B) shows a summary of projections in seven internal company memos and five peer-reviewed publications between 1977 and 2003 (gray lines).
Panel (C) shows a 1977 internally reported graph of the global warming “effect of CO2 on an interglacial scale.” (A) and (B) display averaged historical temperature observations, whereas the historical temperature record in (C) is a smoothed Earth system model simulation of the last 150,000 years.
As these graphs clearly show, oil companies knew since the late 1970s, over 40 years ago, of the climatic effects of CO2 emissions. They could even predict the temperatures since the last ice age. In the 70s, solar and wind energy were much more expensive to produce and use than now, but if we had subsidized their development back then as we have done for decades for the fossil fuel industries, our climatic situation now would be much less precarious. Figure \(\PageIndex{10}\) below shows worldwide fossil fuel subsidies in US $billion and in % global GDP from 2015 to 2020 and projections after that.
Figure \(\PageIndex{10}\): Worldwide subsidizes in US $billion and in % global GDP. Bar graphs are for US$biillons and the circles and triangles for % global GDP. IMF. ttps://www.imf.org/en/Publications/W...bsidies-466004
The subsidies are broken down into explicit subsidies (tax breaks or direct payments to help fossil fuel companies to fund their uncompensated costs) and implicit ones (undercharging for environmental costs of fossil fuel use that the oil companies don't pay). These latter "hidden" costs are passed down to countries, states, and individuals. In 2020, global subsidies were $5.9 trillion or 6.8% of the world's GDP. The explicit subsidies given to fossil fuel companies, about 8% of the total, amounted to $472 billion just in 2020!
"Company executives chose to publicly denigrate climate models, insist there was no scientific consensus on anthropogenic climate change, and claim the science was highly uncertain when their own scientists were telling them the opposite" (ref). They also propagated the myth that the global climate was actually cooling. This is a powerful and unsettling example of disinformation with enormous consequences.
Now that we have seen the big picture, let's look at how carbon moves through various pools of carbon-containing molecules. We have already discussed photosynthesis in great detail in Chapter 20, so we fill focus our attention more on dissolved inorganic carbons (DIC) including species such as HCO3- and CO32-. Another view of the carbon cycle that includes weathering of rocks to produce silicates and bicarbonates, and the formation of shells in the ocean from HCO3-, CO32- and silicates, is shown in Figure \(\PageIndex{10}\).
Figure \(\PageIndex{10}\):
Let's focus on the oceans first. The reversible movement of CO2 from the atmosphere to the oceans, CO2 atm ↔ CO2 ocean, depends on the difference in the partial pressures of CO2 (ΔpCO2) in the atmosphere and surface waters. The reaction is also driven to the right by the removal of CO2 (aq) as it forms carbonic acid (H2CO3), which then forms bicarbonate (HCO3–) and carbonate (CO32–). These coupled reactions chemically buffer ocean water, thus regulating ocean pCO2 and pH.
pCO2 is not homogenous in ocean surface waters and varies with different conditions of current and temperature. CO2 can be more readily released from upwellings of nutrient-rich and warm waters, especially in the tropics. In cold Northern waters and also in the Southern Ocean, where water sinks, it is taken up from the atmosphere (again CO2 is more soluble in cold water).
As we discussed in Chapter 31.1, the ocean chemistry of CO2 determines in large part the levels of atmospheric CO2. The coupled reactions of CO2 in the oceans are shown below.
\begin{equation}
\mathrm{CO}_2(\mathrm{~g}, \mathrm{~atm}) \leftrightarrow \mathrm{CO}_2(\mathrm{aq}, \text { ocean) }
\end{equation}
\begin{equation}
\mathrm{CO}_2(\mathrm{aq} \text {, ocean })+\mathrm{H}_2 \mathrm{O}(\mathrm{I} \text {, ocean }) \leftrightarrow \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{HCO}_3^{-}(\mathrm{aq})
\end{equation}
\begin{equation}
\mathrm{H}_2 \mathrm{O}(\mathrm{I})+\mathrm{HCO}_3^{-}(\mathrm{aq}) \leftrightarrow \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{CO}_3{ }^{2-}(\mathrm{aq} \text {, sparingly soluble })
\end{equation}
These reactions should be familiar to all chemistry students and were presented previously in Chapter 31.1 and in Chapter 2. A significant contributor to ocean bicarbonate is weathering of rocks like limestone. and marble, which are both forms of CaCO3. The relevant reactions are shown below.
\begin{equation}
\begin{aligned}
&\mathrm{CaCO}_3(\mathrm{~s})+\mathrm{H}_2 \mathrm{O} \leftrightarrow \mathrm{Ca}^{2+}(\mathrm{aq})+\mathrm{CO}_3{ }^{2-}(\mathrm{aq}) \\
&\mathrm{CO}_3{ }^{2-}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O} \leftrightarrow \mathrm{HCO}_3{ }^{-}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})
\end{aligned}
\end{equation}
CO2 is nonpolar and not very soluble in water. Either is CO32- in the presence of divalent cations like Ca2+. However HCO3- is and can be considered a "soluble" form of carbon. This soluble form from terrestrial weatherings ends up in rivers and eventually enters the ocean. It is also the form of carbonate that is transferred into cells by anion transporters for eventual shell formation. HCO3- is also a chief regulator of both blood and ocean pH. Weathering is slow compared to anthropogenic emissions of CO2 from fossil fuel use, but it is nevertheless a key player in the carbon cycle and the regulation of ocean pH.
The same weathering reactions on silicate rocks lead to the transfer of silicate ions into rivers and then into the ocean, where they can be taken up by diatoms in the formation of CaSiO4 shells. As the oceans take up more CO2, they become more acidic, which leads to the equivalent of "weathering" of shells of living organisms, leading to their potential death. Silicon is directly underneath carbon in the periodic table so the following simplified reaction is analogous to those we seen with CO2 and its inorganic ions.
\begin{equation}
\mathrm{H}_4 \mathrm{SiO}_4=\mathrm{SiO}_2+2 \mathrm{H}_2 \mathrm{O}
\end{equation}
H4SiO4 is silicic acid.
13C/12C ratios in ice core and ocean sediments
We are now in the position to explore how isotopes of carbon can be used for more than radio- 14C dating, which is quite limited in climate studies. 13C, a stable isotope of carbon, however, is extremely useful because C-13C bond dynamics are influenced by it. Reaction rates are affected by the presence of 13C when C-C bonds are made or cleaved. This isotope effect leads to different 13C/12C ratios in reactants and products, and hence different δ13C values.
Isotopes have a long history in the study of biochemical reactions. The kcat and kcat/KM values for enzyme-catalyzed reactions can be affected if the rate-limiting step involves cleavage or the creation of a C-13C, C-D (deuterium) or C-T (tritium) bond. Substrates labeled with the isotopes have similar transition state energies for the formation/cleavage of a bond involving an isotope, but the ground state vibrational energy for the isotope-substituted atom are proportionately lower, as illustrated in Figure \(\PageIndex{11}\).
Figure \(\PageIndex{11}\): Kinetic Isotope Effects.
This primary kinetic isotope effect leads to higher activation energy for the formation/cleavage of a bond with the isotope. For C-D and C-T bond cleavages that are rate-limiting, the rates are 7X and 16X slower than the cleavage of a C-H bond, respectively. Cleavage or formation of bonds to heavy isotopes of carbon, oxygen, nitrogen, sulfur, and bromine have much smaller isotope effects (between 1.02 and 1.10). The difference in the magnitude of the kinetic isotope effect is directly related to the percentage change in mass. Large effects are seen when hydrogen is replaced with deuterium because the percentage mass change is very large (mass is being doubled). .
Hence the kinetic isotope effect is at play in carbon fixation in photosynthesis, for example. This is evidenced by the observation that the 13C/12C ratios are lower in plants than in the atmosphere, showing that 12CO2 is preferentially "fixed" in the ribulose bisphosphate carboxylase/oxygenase reaction in plants and other photosynthetic organisms. Also, 12CO2, a lighter molecule, has a faster rate of diffusion through the stromata, regulated pores in leaves that facilitate the passage of CO2, O2 and H2O.
In Chapter 31.2, we discussed the use of δ18O values in ice core and ocean core sediments for measuring past CO2 and temperatures.
\[\delta^{18} O=\left[ \dfrac{\left(\dfrac{^{18} O}{^{16} O}\right)_{\text {sample }}}{\left(\dfrac{^{18} O}{^{16} O}\right)_{\text {reference }}}-1\right] * 1000 \nonumber \]
δ18O values for ice core water samples werer easier to interpret than δ18O values for CaCO3 sampls, since the deposition of ice is a simple physical process compared to the complexity of the deposition of CaCO3 in ocean sediments, which depends on chemical reactions and nonequilbrium processes (as described in Chapter 31).
Climate scientists can determine and use δ13C values. An analogous equation for it is shown below.
\(
\delta^{13} C=\left[\dfrac{\left(\frac{13}{12} C\right)_{\text {sample }}}{\left(\frac{13}{12} \mathrm{C}\right)_{\text {reference }}}-1\right] * 1000
\)
As for using δ18O in carbonate samples, using δ13C is more difficult as well. The shells of ocean sediment foraminifera were made from dissolved inorganic carbon (DIC) in the ocean at the time so their δ13C values reflect that. However, shell formation is not a simple equilibrium process since biological shells are formed rapidly so kinetic effects in carbonate and hence isotope fractionation are important. In addition, the biochemistry of shell formation is complicated.
In the open ocean, planktic foraminifera are perhaps the most important marine organism that forms shells given that they produce and export into the ocean about 2.9 Gt CaCO3/yr. Their shells form in a process involving many metastable calcite phases. It starts with a soft template that contains Mg2+ and Na+ ions which play a key role in crystallization. Growth occurs by successive additions of "chambers" to the shell. An F-actin mesh, which forms microtubular structures, leads to the formation of protective envelopes for chamber formation. The layered templates sequester and help control the mineralization of shells and separate bulk sea water for a more intracellular vs extracellular process for biomineralization. Seawater containing minerals becomes vacuolized in a process which for some foraminfera excludes a competing cation, Mg2+. In addition, both Ca2+ and HCO3- transporters are required. This all combines to form an environment low in Mg2+ and supersaturated in Ca2+ and CO32- for calcite formation. The kinetic fractionation of 13C isotopes into shells is also different than for 18O isotopes since the "pool" of oxygen in the oceans is much greater than carbon. Likewise, the δ13C is more location-dependent that the δ18O.
Buried organic matter can also be studied. The δ13C value for buried organic matter depends on primary productivity on land and in the oceans. As mentioned above, autotrophs preferentially take up 12CO2. Heterotrophs that eat them also become enriched in 12C. Hence organisms have negative δ13C values, typically around −25‰, with the number depending on pathways of incorporation and metabolism. Methane in hydrates in the ocean can be either biogenic, made by methanogens, for example, at low temperatures, or thermogenic, made during high-temperature reactions. Biogenic methane has a δ13C of around - 60‰, while thermogenic methane has a value of around −40‰. Terrestrial plants have different δ13 values. δ13C in C4 plants range from -16 to −10‰ while for C3 plants they range from −33 to −24‰.
Changes in δ13C in ice cores and ocean sediments are used in climate studies. Sometimes it's confusing to understand the cause and effect of the changes. This following explanation for changes in the already negative values of δ13C might offer help to those with a chemistry-centric view of biochemistry who struggle with mass balance outside of simple chemical equations.
Under climatic conditions, when there is an abundance of terrestrial plants that lock in and sequester atmospheric 12CO2, the atmosphere becomes depleted in 12CO2 and correspondingly enriched in 13CO2. Hence primary production (fixing of carbon and anabolic metabolism) by photoplankton in the oceans, under robust growing conditions, would sequester more 13C, causing an increase in δ13C (i.e. more positive) values for buried organic and calcite sediments.
During times of mass extinction, when terrestrial plant primary production drops precipitously, the δ13C becomes more negative with the decrease in primary production and release of plant carbon, leaving more 12CO2 in the atmosphere. This drop is called a negative δ13C excursion. When life is robustly favored and carbon is fixed by autotrophs, and the organic carbon resulting from them is eventually buried in sedimentary rocks, the rise in δ13C is called a positive δ13C excursion.
Examples of climatic events accompanied by changes in δ13C.
Late Devonian period
Fossil evidence from the late Devonian, when large terrestrial plants evolved and expanded, is characterized by increases in δ13C.
Paleocene/Eocene Thermal Maximum
We saw in Chapter 31.1 that around 55 MYA, sediment records indicate a spike in temperatures of about 50 F occurring over about a 100K year timeframe. This was accompanied by a dramatic spike in CO2 and a dramatic drop in ocean pH as measured by the loss of deep sea CaCO3 (chalk). This very short time frame is called the Paleocene/Eocene thermal maximum (PETM), which shows very quick spikes (on the geological time scale) can and do occur. Sediment records for this time indicate a large negative δ13C excursion, consistent with a loss of plants with their preferential uptake of 12CO2, leading to an accompanying increase in 12CO2 in the atmosphere.
1500-1650 CE
We examined δ18O values during the Little Ice Ages in Chapter 31.2. What about δ13C values? CO2 and δ13C values from 1000 to 1900 are shown in Figure \(\PageIndex{12}\).
Figure \(\PageIndex{12}\): CO2 and δ13C values from 1000 to 1900. Koch et al. Quaternary Science Reviews, 207, 2019, 13-36. https://doi.org/10.1016/j.quascirev.2018.12.004. CC BY license (http://creativecommons.org/licenses/by/4.0/).
Panel (A) shows the CO2 concentrations recorded in two Antarctic ice cores: Law Dome (grey, MacFarling Meure et al., 2006) and West Antarctic Ice Sheet (WAIS) Divide (blue, Ahn et al., 2012).
Panel (B) shows the carbon isotopic ratios recorded in CO2 from the WAIS Divide ice core (black, Bauska et al., 2015) showing an increased terrestrial carbon uptake over the 16th century (B). The yellow box is the span of the major indigenous depopulation event (1520e1700 CE). Loess smoothed lines for visual aid.
Koch et al have strong evidence to suggest that the cooling after 1510 (area in the yellow box in the above figure) was associated with a dip in CO2 caused by the reforestation of indigenous peoples' land in Meso and South American after epidemics of European disease killed upwards of 90% (around 55 million) of the indigenous peoples. The open and agricultural land reverted back to forests. The diseases included smallpox, measles, influenza, the bubonic plague, and eventually malaria, diphtheria, typhus and cholera. Domesticated farm animals brought from Europe to the Americans led to most of the disease. Along with the death of so many people was a concomitant return of cleared and agricultural lands (about 56 million hectares or 212,000 mi2) to forest and plant growth. This may have led to a 7-10 ppm drop in CO2 in the late 1500s and early 1600s, peaking in 1601 (middle of the yellow box). This decrease in temperature was associated with a small rise (small positive excursion) in the δ13C values, as 12CO2 was preferentially removed from the atmosphere. Global surface air temperatures decreased by around 0.15oC. This "Great Dying" of Indigenous peoples shows the power of humankind to globally alter climate in calamitous ways, even before the use of fossil fuels. The decrease in δ13C values before 1500 was unexplained.
1800-the present
δ13C values can also be used to unequivocally prove that the increase in CO2 since the industrial revolution is from the burning of fossil fuels, which is of biogenic origin and hence have more negative δ13C values. Figure \(\PageIndex{13}\) shows atmospheric CO2 levels in ppm plotted along with δ13C values. There is a perfect correlation between the rise in atmospheric CO2 starting with the industrial revolution with the decrease in the δ13C values over the same time.
Figure \(\PageIndex{13}\): CO2 concentration (black circles) and the δ13C (brown circles) from 1000 to 2010. Rubino et al. Journal of Geophysical Research: Atmospheres. https://doi.org/10.1002/jgrd.50668. With permission (Copyright Clearance Center)
Summary
In the first three sections of Chapter 31 (31.1, 31.2, and this one), we have introduced the basics of climate change, and how climate scientists obtain, analyze and interpret climate data. We emphasized the scientific rigor by which they do that and offered a detailed analysis of the use of isotopes to document past and present changes in climate, Finally, we offered models to predict and mitigate future climate changes. After reading this material, you should be enabled to discuss climate change with others from a sound and valid position. More importantly for this book, you will have a better knowledge base and understanding for the rest of the chapter sections, which will address specific topics in "biochemistry and climate change".
- The carbon cycle is the process by which carbon moves through the Earth's systems, including the atmosphere, oceans, and biosphere.
- The carbon cycle is driven by the exchange of carbon between different reservoirs, such as the atmosphere, oceans, and living organisms.
- The main processes involved in the carbon cycle include photosynthesis, respiration, and the formation and weathering of rocks.
- Human activities, such as burning fossil fuels and deforestation, have significantly increased the amount of carbon dioxide (CO2) in the atmosphere, disrupting the natural balance of the carbon cycle.
- The increase in atmospheric CO2 is the primary driver of climate change, as it causes the greenhouse effect, trapping heat in the atmosphere and warming the Earth's surface.
- The ocean also plays a critical role in the carbon cycle, as it acts as a sink for CO2, absorbing about 25% of the CO2 emitted by human activities.
- The acidification of the ocean caused by the uptake of CO2 is having a significant impact on marine ecosystems, altering the chemistry of seawater and making it more difficult for some organisms to build and maintain their shells and skeletons.
- Understanding the carbon cycle and carbon chemistry is crucial for understanding the causes and impacts of climate change and for developing strategies to mitigate and adapt to its effects.