29.9: Immunometabolism- Cellular Metabolism Turns Immune Regulator
- Page ID
- 53919
Róisín M. Loftus and David K. Finlay. Immunometabolism: Cellular Metabolism Turns Immune Regulator. Journal of Biological Chemistry. MINIREVIEWS| VOLUME 291, ISSUE 1, P1-10, JANUARY 01, 2016. https://www.jbc.org/article/S0021-92...233-5/fulltext
Abstract
Immune cells are highly dynamic in terms of their growth, proliferation, and effector functions as they respond to immunological challenges. Different immune cells can adopt distinct metabolic configurations that allow the cell to balance its requirements for energy, molecular biosynthesis, and longevity. However, in addition to facilitating immune cell responses, it is now becoming clear that cellular metabolism has direct roles in regulating immune cell function. This review article describes the distinct metabolic signatures of key immune cells, explains how these metabolic setups facilitate immune function, and discusses the emerging evidence that intracellular metabolism has an integral role in controlling immune responses.
Metabolic Challenges Facing Immune Cells
During the course of an immune response, immune cells can traverse multiple tissues containing diverse conditions of nutrient and oxygen availability. Additionally, in response to activation, immune cells often dramatically change their functional activities; a lymphocyte transforms from a relatively inert cell to a cell engaging in robust growth and proliferation, often producing large amounts of effector molecules such as cytokines. These microenvironmental and functional alterations represent significant metabolic stresses that are efficiently managed by immune cells due their ability to dynamically reprogram their cellular metabolism.
Inflammatory Microenvironments
Most normal tissue is well vascularized and replete with nutrients and oxygen. However, during an immune response, conditions in the local immune microenvironment can often be significantly less accommodating due to competition for nutrients. For example, tumor cells have a prodigious appetite for glucose and other nutrients. As a result, the microenvironment within solid tumors can become depleted of glucose, resulting in decreased rates of glycolysis in tumor infiltrating lymphocytes (1, 2, 3). Bacterial infections can also compete for nutrients with immune cells. Infection with Staphylococcus aureus, a common human pathogen, can result in localized tissue hypoxia due to elevated levels of oxygen consumption by the invading bacteria. As glucose is a key fuel for this bacteria, the levels of glucose available to immune cells will also be reduced (4). Viral infection can also result in a decrease in the amount of glucose that is available to infiltrating immune cells; viruses can reprogram infected cells to up-regulate glucose uptake and metabolism to facilitate viral replication (5, 6, 7). Additionally, various cells at sites of inflammation can release enzymes that consume nutrients in the local microenvironment, including arginase and indoleamine-2,3-dioxygenase, which deplete arginine and tryptophan, respectively (1). Inflammatory sites can also become hypoxic due to the pronounced influx of inflammatory cells such as neutrophils and monocytes (8).
Dynamic Changes in Cellular Function
Immune activation is accompanied by substantial changes in cellular activities, such as those accompanying T cell activation. Naïve T cells are long-lived, relatively inert, exhibit low levels of cellular biosynthesis, and primarily require ATP to meet cellular demands (Fig. 1A). Following activation, T cells undergo substantial changes in function and engage in robust cellular growth and rapid cellular proliferation (9). Essential in supporting these cellular activities is the provision of sufficient biomolecules (amino acids, nucleotides, lipids) for the biosynthesis of new cellular components. Therefore, in activated T cells, the objectives of cellular metabolism have shifted from primarily generating ATP to the generation of sufficient ATP plus large amounts of biomolecules for the generation of biomass (10). Therefore, immune cells adapt their cellular metabolism to accommodate altered functional outputs.
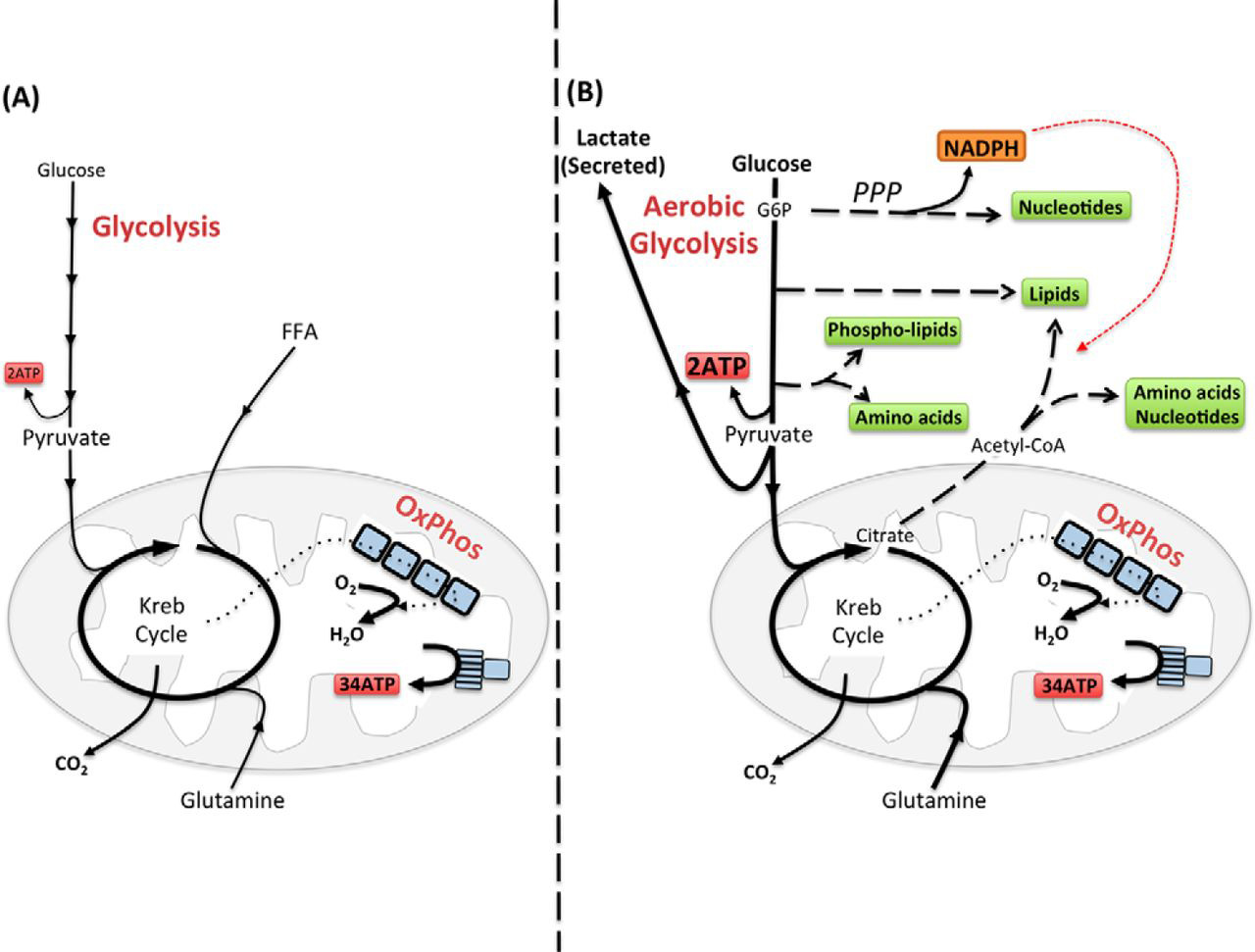
FIGURE 1.Configuring metabolism to match immune cell function. A, ATP is the key molecule that provides energy for cellular processes. Maintaining cellular ATP levels is essential for bioenergetic homeostasis and cell survival. Glucose, a key fuel source for mammalian cells, can be metabolized via two integrated metabolic pathways, glycolysis and OxPhos, that efficiently convert this simple sugar glucose into ATP. Glycolysis converts glucose to pyruvate through a series of enzymatic steps that occur in the cytosol, generating two molecules of ATP. Following its transportation into the mitochondria, pyruvate is further metabolized to CO2 by the Krebs cycle, which drives OxPhos and the translocation of protons across the mitochondrial inner membrane. The resulting proton gradient drives the enzyme ATP synthase, converting ADP to ATP, generating up to 34 ATP per molecule of glucose. In addition to the breakdown of glucose via glycolysis, cells have the ability to metabolize alternative substrates, such as lipids and glutamine, which feed into the Krebs cycle and drive OxPhos. Fatty acid β-oxidation and glutaminolysis replenish the Krebs cycle intermediates acetyl-CoA and α-ketoglutarate, respectively, thereby fueling OxPhos and the efficient generation of cellular ATP. B, aerobic glycolysis supports biosynthetic processes of the cell as it allows the uptake of larger amounts of glucose and the maintenance of elevated glycolytic flux. Glycolytic intermediates are then diverted into various pathways for the synthesis of biomolecules that support biosynthetic processes. For instance, glucose 6-phosphate (G6P), generated by the first step in glycolysis, can feed into the pentose phosphate pathway (PPP) to support nucleotide synthesis. This pathway also generates NADPH, a cofactor that is essential for various biosynthetic processes including lipid synthesis. Glucose can also be converted into cytoplasmic acetyl-CoA via citrate in the Krebs cycle for the production of cholesterol and fatty acids for lipid synthesis. Other glycolytic intermediates can also be converted into biomolecules used for protein and lipid synthesis. During aerobic glycolysis, a significant proportion of pyruvate is also converted to lactate and secreted from the cell. Although aerobic glycolysis is an inefficient way to generate ATP (generating only two ATP molecules per glucose) due to the high rates of flux through the pathway, the rate of ATP production can be sufficient to maintain energy homeostasis even when mitochondrial ATP synthesis is impaired. Alternative fuels including glutamine feed into the Krebs cycle and can also supply biomolecules for biosynthetic processes under certain conditions.
Configuring Metabolism for Biosynthesis, Inflammation, and Longevity
Aerobic Glycolysis for Cellular Biosynthesis
A common feature of pro-inflammatory immune cells is that they adopt a distinct metabolic signature termed “aerobic glycolysis” to support cellular biosynthetic processes: that is, glucose metabolized to lactate in the presence of abundant oxygen (Fig. 1B). Aerobic glycolysis is adopted by cells engaging in robust growth and proliferation because it provides the biosynthetic precursors that are essential for the synthesis of nucleotides, amino acids, and lipids (10). Many intermediates of the glycolytic pathway act as a source of carbon that feeds into a range of biosynthetic pathways (Fig. 1B). Therefore, for cells engaged in aerobic glycolysis, the function of glucose is not just as a fuel to generate energy but also as a source of carbon that can be used for biosynthetic purposes (11). Hence, aerobic glycolysis provides immune cells with the components needed to facilitate proliferation and the synthesis of inflammatory molecules.
Aerobic Glycolysis in Activated Lymphocytes
Upon stimulation through antigen or cytokine receptors, lymphocytes increase the rates of both glycolysis and OxPhos (Fig. 2) (10). Although glucose is an essential fuel during T cell activation, glutamine is also important, and effector T cell differentiation is impaired when the supply of glutamine is disrupted (9, 14, 15). T cells that differentiate into effector subsets maintain aerobic glycolysis in response to various cytokines (16). In contrast, FoxP3+ regulatory T cells (Tregs) switch to low levels of glycolysis and preferentially use oxidative metabolism (17). However, another type of regulatory T cell, FoxP3− regulatory T cells (Tr1), maintains elevated glycolysis similar to effector T cells (18). Although many of the functions of Tr1 cells overlap with those of Tregs, others are unique to Tr1 cells including granzyme/perforin-mediated cytolysis of target cells. Therefore, perhaps the distinct metabolic characteristics of these regulatory cells reflect the different mechanisms through which they regulate T cell responses. Similarly, B lymphocytes and NK cells also increase rates of glycolysis and OxPhos in response to various stimuli (19,20, 21,22). However, as metabolic analyses of B lymphocytes have all been performed using in vitro stimulated splenic B cells, the metabolic profile of distinct B cell subsets is currently unknown. Similarly, the metabolic signatures of distinct NK subsets, or indeed other innate lymphoid cells, also remain to be characterized.
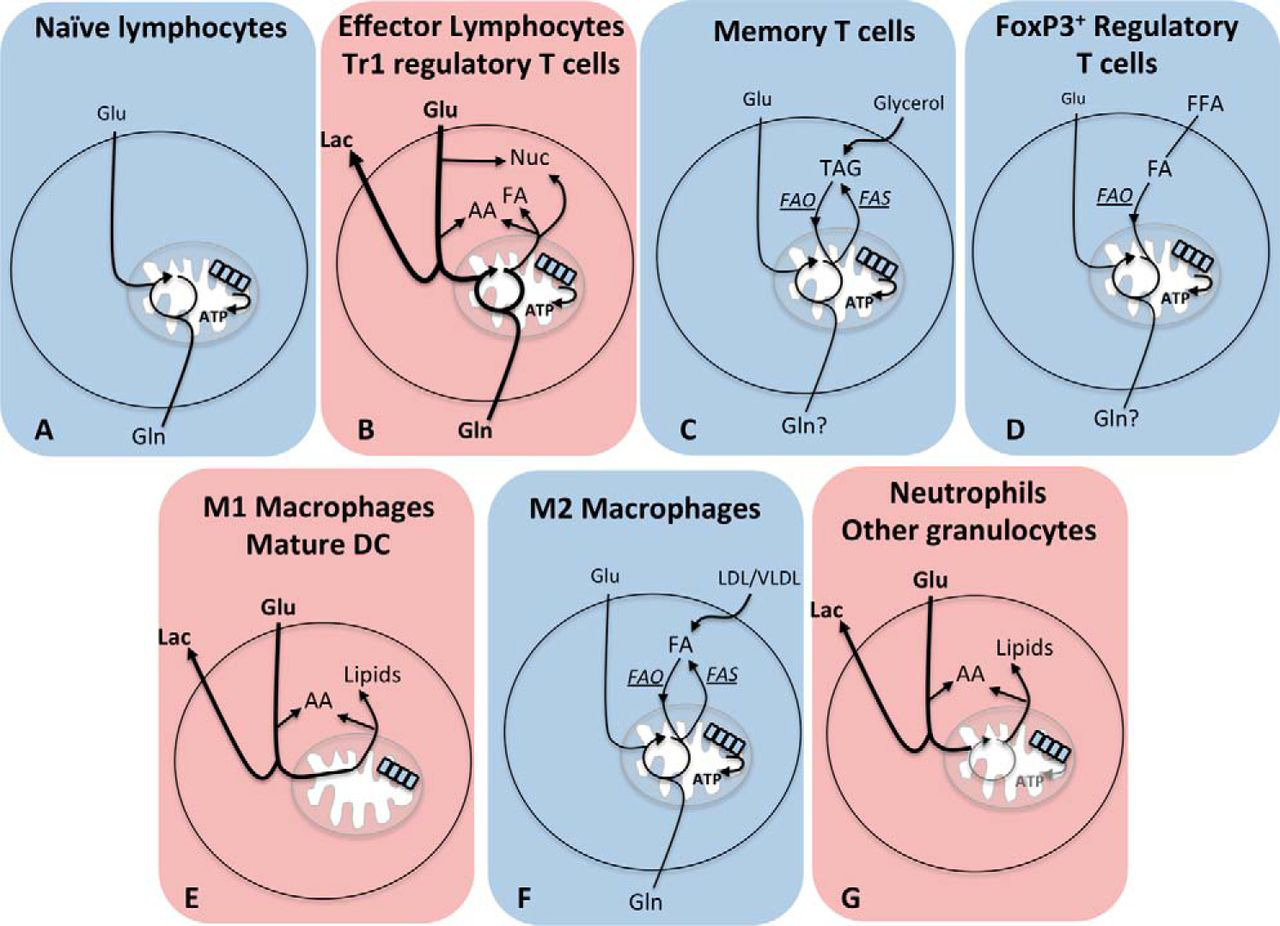
FIGURE 2.Distinct metabolic configurations of different immune cell subsets. Blue panels represent cells with oxidative metabolism, and red panels represent cells with glycolytic metabolism. A, naïve T cells use glucose and glutamine and OxPhos. B, effector lymphocytes and Tr1 regulatory T cells have high rates of both glycolysis and OxPhos, metabolize glucose to lactate, and use metabolic intermediates to support biosynthetic processes. Nuc, nucleotides; FA, fatty acids; AA, amino acids; Lac, Lactate. C, memory T cells use glucose to generate mitochondrial citrate, which is exported into the cytosol to support lipid synthesis. These de novo synthesized fatty acids are used with imported glycerol to generate and store TAGs. OxPhos is fueled by acetyl-CoA generated following β-oxidation of these TAGs. FAO, fatty acid oxidation; FAS, fatty acid synthesis. D, FoxP3+ regulatory T cells use exogenously derived fatty acids metabolized by β-oxidation to support OxPhos. E, M1 macrophages and mature DC engage aerobic glycolysis for ATP synthesis and to support biosynthesis while also inactivating OxPhos. F, M2 macrophage metabolism is characterized by fatty acid β-oxidation and OxPhos. β-Oxidation is fueled by lipids that are scavenged from the external microenvironment and also by lipids generated by de novo fatty acid synthesis. G, neutrophils are highly glycolytic with few functional mitochondrial and very low rates of OxPhos.
Although the exact molecular mechanisms controlling glycolytic metabolism are not universal for all lymphocyte subsets, it is clear that mammalian target of rapamycin (mTOR) has a fundamental role (10, 23). mTOR complex 1 (mTORC1) activity is essential for the initial induction of glycolysis in T cells and is also required to maintain aerobic glycolysis in effector T cells subsets (9, 23, 24). The data also suggest that mTORC1 has an important role for cytokine-induced glycolysis in NK cells (22). A number of transcription factors are involved in glycolytic reprogramming of T cells including both hypoxia-inducible factor 1α (HIF1α) and c-Myc (9, 25, 26). In B cells, c-Myc but not HIF1α is important for the glycolytic response (21). HIF1α and c-Myc directly bind the promoters of an array of genes, notably those of glycolytic enzymes and glucose transporters.
Aerobic Glycolysis in Myeloid Cells
Unlike lymphocytes, mature myeloid cells tend to be non-proliferative and so have substantially different metabolic requirements. Activated M1 macrophages, dendritic cells (DC), and granulocytes are all highly glycolytic with little or no flux through OxPhos (27,28, 29, 30, 31,32). In activated M1 macrophages and DC, OxPhos is inactivated following inducible NOS-dependent nitric oxide production, which directly inhibits oxidative phosphorylation (33, 34). In these cells, the Krebs cycle is no longer cycling, which allows the repurposing of Krebs cycle enzymes to generate molecules that are important for proinflammatory functions (27, 33). M1 macrophages generate high levels of the Krebs cycle metabolite succinate, which can lead to increased HIF1α activity and sustained IL1β production (27). Levels of citrate are also elevated and are used to generate the antimicrobial metabolite itaconic acid that inhibits the growth of bacteria such as Salmonella enterica and Mycobacterium tuberculosis (34, 35). The metabolic changes following DC activation occur in two phases and result in a metabolic switch from fatty acid β-oxidation and OxPhos to glycolysis (33). An initial increase in glycolysis occurs within minutes of DC activation to support de novo lipid biosynthesis, facilitating the expansion of endoplasmic reticulum and Golgi apparatus and increasing the biosynthetic capacity that is essential for mature DC function (36). Over the course of 18 h, activated DC sustain elevated glycolysis and inactivate OxPhos (33). This metabolic shift is important in regulating DC-induced T cell responses, in part due to the fact that it impacts upon DC lifespan and thus the duration over which DC can activate T cells (37, 38). The metabolism of granulocytes is best characterized for neutrophils, which rely almost entirely on glycolysis and exhibit very low levels of OxPhos (28, 29,30, 39). Neutrophil effector functions, including the formation of neutrophil extracellular traps, require mTORC1/HIF1α signaling and glucose metabolism (29, 30, 39, 40,41). Although the metabolism of other granulocytes such as basophils and eosinophils remains poorly characterized, there is some evidence that these cells are also glycolytic and rely upon metabolic regulators such as HIF1α to maintain glycolysis and normal function (42). For instance, HIF1α accumulation upon basophil activation was shown to be required for VEGF and IL4 production (42).
Oxidative Cellular Metabolism in Naïve Lymphocytes and Memory T Cells
As previously mentioned, naïve lymphocytes are relatively inert cells with limited biosynthetic demands, and so ATP alone is relatively sufficient to sustain these cells. Given that these cells reside in well oxygenated tissues, oxidative metabolism is a consistent and efficient way to meet cellular metabolic demands. Memory cells generated during the course of an immune response share many of the same characteristics of naïve lymphocytes; they are long-lived, relatively inert cells with limited biosynthetic demands. As nothing is known regarding the metabolism of memory B cells, only memory T cells will be considered here. The key distinction between naïve and memory T cells is the rapid recall responses characteristic of memory T cells when compared with primary T cell responses. Although both naïve and memory T cells adopt oxidative metabolism, there are key differences in the metabolic configurations of these cells that contribute to rapid memory T cell recall responses. Memory T cells predominantly use fatty acid β-oxidation to generate acetyl-CoA to fuel OxPhos (43) (Fig. 2). β-Oxidation is an efficient method for generating ATP with each fatty acid molecule generating significantly more ATP (about 106 ATP/molecule of palmitate) when compared with one molecule of glucose (about 36 ATP/molecule of glucose). Indeed, fatty acid oxidation is essential for rapid memory T cell responses (43). Interestingly, these fatty acids are not taken up from the surrounding microenvironment, but rather memory T cells use glucose and glycolysis to generate citrate for de novo fatty acid synthesis and the generation and storage of triacylglycerides (TAGs) (44, 45). These endogenously derived TAGs are then broken down by β-oxidation in the mitochondria to generate acetyl-CoA to fuel OxPhos (45). From a bioenergetics standpoint, this would seem like an inefficient mechanism to fuel OxPhos as fatty acid synthesis utilizes both ATP and NADPH. Nonetheless, this seemingly futile cycle of fatty acid synthesis and fatty acid oxidation is important for memory T cell survival (44, 45). This approach may be taken by memory T cells, for which long term survival is of utmost importance, as glucose levels are stringently controlled in the blood, making glucose a more dependable fuel source than fatty acids, whose levels can vary in different tissues. Another advantage of this cycle of fatty acid synthesis and oxidation may be that it allows the cell to concurrently engage both glycolysis and OxPhos, thus maintaining the machinery required for rapid induction of metabolic flux through these pathways upon antigen recognition and so facilitating rapid functional responses. Indeed, memory T cells can induce rates of glycolysis much more rapidly and robustly than naïve T cells (43, 46).
Oxidative Cellular Metabolism in Cells with Significant Biosynthetic Output
FoxP3+ Tregs also primarily engage in oxidative metabolism, but in contrast to naïve lymphocytes and memory T cells, FoxP3+ Tregs are not inert cells and are in fact producing relatively large quantities of biomolecules (17, 47). Tregs make immunosuppressive cytokines IL10 and TGFβ and can also engage in cellular proliferation in response to IL2. In this respect, M2 macrophages are similar to FoxP3+ Tregs; M2 macrophages engage in oxidative metabolism and yet have significant biosynthetic outputs. M2 macrophages have roles in tissue repair and secrete anti-inflammatory cytokines, growth factors, and factors involved in tissue remodeling (48). Tregs and M2 macrophages oxidize both glucose and fatty acids in the mitochondria to sustain OxPhos (17, 49,50, 51,52). In contrast to memory T cells, Tregs fuel β-oxidation and the Krebs cycle using exogenously derived fatty acids. Meanwhile, in M2 macrophages, there is evidence that both exogenously derived lipids scavenged from the microenvironment and de novo synthesized lipids fuel β-oxidation and OxPhos (52). It is likely that Tregs and M2 macrophages use glutamine metabolites to sustain cellular biosynthetic processes (Fig. 1) (53). Indeed, M2 macrophages have increased glutamine metabolism when compared with M1 macrophages (34). Additionally, given that M2 macrophages are professional scavengers of apoptotic debris, it is tempting to speculate that M2 macrophages sustain cellular biosynthesis using biomolecules scavenged from the surrounding microenvironment (48, 52).
Oxidative Metabolism Supports Immune Cell Longevity
Controlling the longevity of immune cells is an important aspect of a healthy immune system. For example, a long lifespan (years) is essential for naïve and memory T cells to maintain functional primary and recall T cell responses. In contrast, it is crucial that upon resolution of a viral infection, the large population of CTL undergoes apoptosis as these effector T cells have the potential to cause significant immunopathology (54). Therefore, CTL have a short lifespan of days to weeks. Similarly, differences in lifespan are apparent in different subsets of macrophages. M1 macrophages are short-lived and are a key component of the innate immune system that forms the first line of defense occurring within hours to days of an immunological challenge. In contrast, M2 macrophages are longer-lived as they have important roles within the resolution phase and in tissue repair and remodeling. Strikingly, the cellular metabolic signature of an immune cell corresponds to the longevity of the cell; aerobic glycolysis is characteristic of short-lived immune cells, whereas oxidative metabolism is characteristic of long-lived cells (Fig. 2).
It is perhaps unsurprising that OxPhos is important for longevity in immune cells given the importance of mitochondrial membrane potential in controlling the induction of apoptosis. Certainly, in activated DC, preserving OxPhos results in an increased cellular lifespan (38). Moreover, in macrophages, switching cellular metabolism from glycolysis to oxidative metabolism promotes a shift from short-lived M1 macrophages to longer-lived M2 macrophages (50). In addition, manipulating glycolytic versus oxidative metabolism impacts upon the formation of long-lived memory T cells; inhibiting glycolysis promotes memory T cell formation, whereas inhibiting fatty acid oxidation-dependent OxPhos represses memory T cell formation (55, 56). These reports are consistent with a number of other studies that also support the notion that promoting oxidative phosphorylation enhances cell survival and lifespan (57, 58,59). On the other hand, there are also numerous reports on a variety of cell types showing that manipulating glycolytic metabolism has profound impacts upon cellular viability (60,61, 62, 63,64). Growth factors that promote elevated levels of cellular glycolysis also have the consequence of making that cell highly dependent on continued growth factor signaling and glycolysis for survival (64). This provides an elegant mechanism for terminating effector T cell responses. For instance, glycolytic metabolism in CD8+ CTL is sustained by IL2, and upon IL2 withdrawal, as will occur upon resolution of a viral infection, glycolytic metabolism is rapidly lost and the CTL will die (24, 65).
Metabolic Control of Immune Cell Function
Metabolic Enzymes or Regulators Controlling Immune Cell Function
Cellular metabolism is crucial for facilitating immune cell functions, but in addition, there is emerging evidence that metabolic enzymes and regulators can also have a direct role in controlling immune cell functions. For instance, in CD4 T cells, GAPDH has been described to bind to the 3′-UTR of IFNγ and IL2 mRNA and inhibit translation (66). This function of GAPDH is perhaps unsurprising due to the numerous reports describing RNA binding activities for GAPDH over the past two decades (67, 68, 69,70). Indeed, in myeloid cells, GAPDH is a component of the IFNγ-activated inhibitor of translation (GAIT) complex that binds defined 3′-UTR elements within a family of inflammatory mRNAs and suppresses their translation (71). Importantly, GAPDH functions in glycolysis and mRNA binding are likely to be mutually exclusive so that in glycolytic cells, GAPDH is preferentially engaged in glycolysis, and thus the translation of IFNγ and IL2 mRNA is unconstrained. This mechanism provides a direct link between rates of glycolysis and the expression of important immunological effector molecules. Intriguingly, it appears that many other metabolic enzymes can bind to mRNA molecules including numerous glycolytic enzymes, Krebs cycle enzymes, and enzymes involved in other metabolic pathways (72). Although the specific mRNA transcripts that these metabolic enzymes bind to still have to be identified, this study highlights the abundant potential for cellular metabolism to directly impact upon cellular functions.
Various metabolic regulators that evolved to control cellular metabolic pathways have since acquired roles in directly controlling immune cell function. The glycolytic regulator HIF1α also promotes the expression of IL1β in M1 macrophages and programmed death ligand-1 (PD-L1), a ligand for the immune checkpoint receptor PD-1, on various myeloid cells (27, 73). The aryl-hydrocarbon receptor (AhR), which together with HIF1α controls glycolytic metabolism in Tr1 regulatory T cells, also directly regulates T cell responses. AhR promotes Th17 differentiation, while inhibiting Treg differentiation, and is required for the production of the Th17 cytokines IL17 and IL22 (74, 75, 76). Additionally, AhR is important for Tr1 regulatory T cell differentiation, directly promoting the expression of IL10 and IL21 (18, 77). The transcription factor sterol regulatory element-binding protein (Srebp), a central regulator fatty acid and cholesterol synthesis, has dual roles in controlling T cell metabolism and directly controlling genes required for immune function. CD8+ T cells lacking Srebp activity fail to undergo metabolic reprogramming and blastogenesis and do not mount a functional T cell response (78). In CD4+ T cells, the Srebp1c isoform is involved in Th17 differentiation and directly binds to the IL17 promotor to inhibit AhR-induced IL17 expression (79). Moreover, the Srebp1a isoform is required for pro-inflammatory functions in myeloid cells, including IL1β production, as it promotes the expression of a key component of the inflammasome, Nlrp1 (80). Therefore, there is growing evidence that multiple important regulators of cellular metabolism have additional functions in directly controlling immune responses.
Metabolites Controlling Immune Cell Function
Distinct metabolic configurations will result in different levels of metabolites that can directly impact upon cellular function. It has recently been shown that the glycolytic intermediate phosphoenolpyruvate is important in sustaining T cell receptor (TCR) signaling and T cell effector functions. Phosphoenolpyruvate inhibits Ca2+ re-uptake into the endoplasmic reticulum, thus sustaining nuclear factor of activated T-cells (NFAT) signaling (2). Mitochondrial reactive oxygen species generated as a side product of OxPhos are also important for optimal TCR signal transduction. T cells that cannot produce mitochondrial reactive oxygen species fail to activate nuclear NFAT, produce IL2, or engage in proliferative expansion (81). In M1 macrophages, the levels of Krebs cycle metabolites are substantially altered, leading to dramatically elevated levels of succinate, the stabilization of HIF1α, and prolonged production of IL1β (27, 34). Succinate can stabilize HIF1α by inhibiting the α-ketoglutarate-dependent prolyl-hydroxylases responsible for tagging HIF1α for proteasomal degradation (27, 82, 83). Indeed, succinate can inhibit other α-ketoglutarate-dependent enzymes that can impact upon immune cells due their roles in controlling cellular epigenetics, namely TET2 DNA hydroxylates and Jumonji C (JmjC) domain-containing histone demethylases (discussed further below) (84, 85). Succinate can act as a signaling molecule that acts through the receptor SUCNR1 and can also be used as a substrate for the post-translational modification of proteins (that is, succinylation) (86). Succinate acting through SUCNR1 impacts upon DC functions and also induces DC chemotaxis to enhance DC-induced T cell responses (87). Numerous metabolic enzymes are succinylated on lysine residues, but at present, it is not clear whether this modification impacts upon the regulation of immune responses (86). Citrate levels are also elevated in M1 macrophages, and this metabolite is important for the production of various proinflammatory molecules: nitric oxide, reactive oxygen species, and prostaglandins (27, 88).
Cellular metabolites are also important substrates for various enzymes involved in the epigenetic control of gene expression via covalent modification of DNA and histones. Given that the distinct metabolic configurations that characterize immune cells result in different levels of these cellular metabolites, it follows that the epigenetic control of gene expression will differ in parallel with differences in metabolism. For example, TET family enzymes, which oxidize methylcytosine, leading to DNA demethylation, and JmjC domain-containing histone demethylases both require α-ketoglutarate as a substrate and are both inhibited by succinate (Fig. 3). Indeed, TET2 has recently been shown to regulate the expression of IFNγ, IL17a, and IL10 in Th1 and Th17 cells (89). Jmjd3 has been shown to be of particular importance in controlling gene expression in LPS-stimulated macrophages (90). Acetylation of histones is another post-translational modification that impacts on DNA structure and gene expression. Acetylation of histones by histone acetyl transferases (HATs) requires acetyl-CoA, which is supplied via the export of mitochondrial citrate (Fig. 3). Indeed, there is evidence in yeast that the concentration of acetyl-CoA is important for histone acetylation (91). Histone acetylation levels are also controlled by the rate of deacetylation. The activity of sirtuin histone deacetylases is linked to cellular metabolism as these deacetylases are sensitive to the ratio of oxidized NAD+ to reduced NADH, which is affected by the balance of glycolysis and OxPhos (92). Oxidized NAD+ is an essential substrate for sirtuins, whereas reduced NADH acts to inhibit sirtuin activity (Fig. 3) (93). In fact, sirtuins can also deacetylate targets other than histones, which are important in immune regulation. For example, Sirt1 deacetylates FoxP3 to inhibit Treg responses and RORγt to promote Th17 responses (94,95, 96,97). Additionally, sirtuins can also have a negative impact upon inflammatory responses, in part through inhibition of NFκB activity (98, 99). Although there are numerous studies suggesting that cellular metabolism impacts upon epigenetic programming of immune cells to affect immune cell fate and function, the best evidence of this comes from a study of trained immunity in macrophages. Cheng et al. (92) elegantly demonstrated that mTORC1/HIF1α-stimulated glycolysis is required for changes in the epigenome of human or murine myeloid cells that provides enhanced nonspecific protection from secondary infections. Therefore, it is clear that metabolites can impact directly on immune cell function, and it is likely that further examples of this will be revealed as the field of immunometabolism progresses.
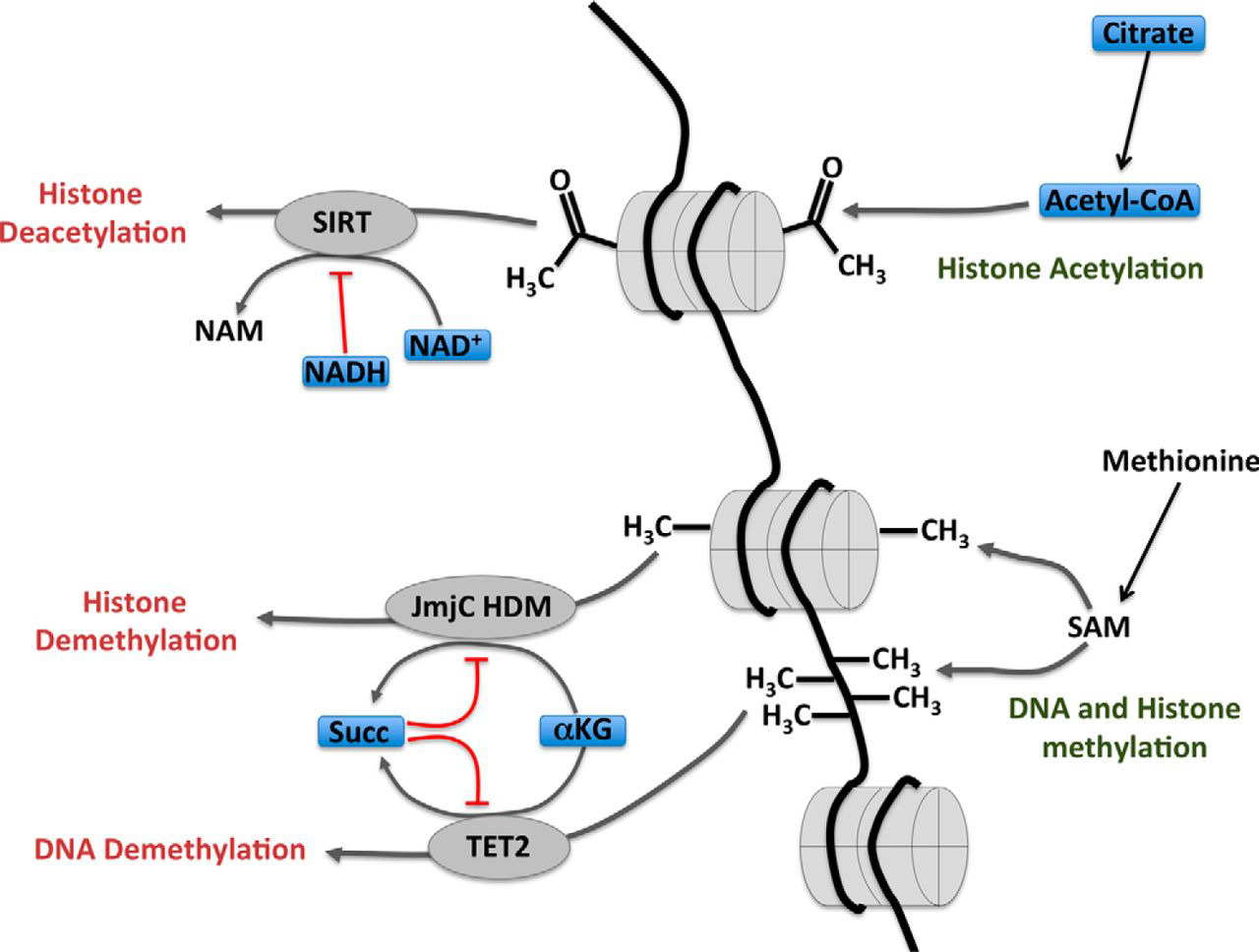
FIGURE 3.Links between cellular metabolism and epigenetic modifications. Histone deacetylation by sirtuin (SIRT) family members requires NAD+ as a substrate, and the activity of these enzymes is inhibited by NADH. The balance of oxidized NAD+ and reduced NADH is affected by levels of glycolysis and OxPhos. Methylation of DNA and histones is controlled by the rates of methylation and demethylation. The activities of JmjC domain-containing histone demethylases and the TET2 hydroxylase lead to histone and DNA demethylation, respectively, and can be regulated by Krebs cycle intermediates α-ketoglutarate (α-KG) and succinate (Succ). α-Ketoglutarate is a substrate for these enzymes, and succinate acts as an inhibitor. NAM, nicotinamide; HDM, histone demethylase; SAM, S-adenosylmethionine.
Immune Metabolism Relays External Signals to Regulate Immune Cell Function
The data now support an important role for cellular metabolism in controlling the function of immune cells. Given that metabolic regulators and pathways are acutely sensitive to external levels of nutrients, oxygen, and growth factors, cellular metabolism represents a means to relay information from the local microenvironment to modulate immune cell function accordingly. Nutrients such as glucose, glutamine, and fatty acids that directly supply metabolic pathways also regulate the activity of important regulators of immune metabolism and function including mTORC1, HIF1α, and Srebp. Other nutrients are important for providing the substrates for enzymes that impact upon immune cell function. For example, methionine, which is an essential amino acid and so must be imported into the cell, is used to generate S-adenosylmethionine for epigenetic methylation of DNA and histones. Although most studies have focused on how activating immune receptors affect cellular metabolism, it is now becoming apparent that ligation of inhibitory receptors also alters metabolic pathways. Recent research has demonstrated that ligation of the inhibitory receptors PD-1 and CTLA-4 expressed on human CD4 T cells has pronounced effects on cellular metabolism, inhibiting aerobic glycolysis, and in the case of PD-1, promoting fatty acid oxidation (100). These data suggest that the inhibitory actions of these receptors may be mediated, at least in part, due to changes in cellular metabolism.
Final Comments
The emerging data now argue that metabolism has duel roles in immune cells to facilitate requirements for energy and biosynthesis and to directly regulate immune cell functions. There are likely to be numerous opportunities for novel therapeutic strategies that modulate this metabolic regulatory axis.
References
-
- Hirayama A.
- Kami K.
- Sugimoto M.
- Sugawara M.
- Toki N.
- Onozuka H.
- Kinoshita T.
- Saito N.
- Ochiai A.
- Tomita M.
- Esumi H.
- Soga T.
Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry.
Cancer Res. 2009; 69: 4918-4925View in Article -
- Ho P.C.
- Bihuniak J.D.
- Macintyre A.N.
- Staron M.
- Liu X.
- Amezquita R.
- Tsui Y.C.
- Cui G.
- Micevic G.
- Perales J.C.
- Kleinstein S.H.
- Abel E.D.
- Insogna K.L.
- Feske S.
- Locasale J.W.
- Bosenberg M.W.
- Rathmell J.C.
- Kaech S.M.
Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses.
Cell. 2015; 162: 1217-1228View in Article -
- Chang C.H.
- Qiu J.
- O'Sullivan D.
- Buck M.D.
- Noguchi T.
- Curtis J.D.
- Chen Q.
- Gindin M.
- Gubin M.M.
- van der Windt G.J.
- Tonc E.
- Schreiber R.D.
- Pearce E.J.
- Pearce E.L.
Metabolic competition in the tumor microenvironment is a driver of cancer progression.
Cell. 2015; 162: 1229-1241View in Article -
- Vitko N.P.
- Spahich N.A.
- Richardson A.R.
Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus.
MBio. 2015; 6: e00045View in Article -
- Ripoli M.
- D'Aprile A.
- Quarato G.
- Sarasin-Filipowicz M.
- Gouttenoire J.
- Scrima R.
- Cela O.
- Boffoli D.
- Heim M.H.
- Moradpour D.
- Capitanio N.
- Piccoli C.
Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 α-mediated glycolytic adaptation.
J. Virol. 2010; 84: 647-660View in Article -
- Yu Y.
- Maguire T.G.
- Alwine J.C.
Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection.
J. Virol. 2011; 85: 1573-1580View in Article -
- Thai M.
- Graham N.A.
- Braas D.
- Nehil M.
- Komisopoulou E.
- Kurdistani S.K.
- McCormick F.
- Graeber T.G.
- Christofk H.R.
Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication.
Cell Metab. 2014; 19: 694-701View in Article -
- Taylor C.T.
- Colgan S.P.
Hypoxia and gastrointestinal disease.
J. Mol. Med. (Berl.). 2007; 85: 1295-1300View in Article -
- Wang R.
- Dillon C.P.
- Shi L.Z.
- Milasta S.
- Carter R.
- Finkelstein D.
- McCormick L.L.
- Fitzgerald P.
- Chi H.
- Munger J.
- Green D.R.
The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation.
Immunity. 2011; 35: 871-882View in Article -
- Donnelly R.P.
- Finlay D.K.
Glucose, glycolysis and lymphocyte responses.
Mol. Immunol. 2015; (10.1016/j.molimm.2015.07.034)View in Article -
- Vander Heiden M.G.
- Cantley L.C.
- Thompson C.B.
Understanding the Warburg effect: the metabolic requirements of cell proliferation.
Science. 2009; 324: 1029-1033View in Article -
- Macintyre A.N.
- Gerriets V.A.
- Nichols A.G.
- Michalek R.D.
- Rudolph M.C.
- Deoliveira D.
- Anderson S.M.
- Abel E.D.
- Chen B.J.
- Hale L.P.
- Rathmell J.C.
The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function.
Cell Metab. 2014; 20: 61-72View in Article -
- Blagih J.
- Coulombe F.
- Vincent E.E.
- Dupuy F.
- Galicia-Vázquez G.
- Yurchenko E.
- Raissi T.C.
- van der Windt G.J.
- Viollet B.
- Pearce E.L.
- Pelletier J.
- Piccirillo C.A.
- Krawczyk C.M.
- Divangahi M.
- Jones R.G.
The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo.
Immunity. 2015; 42: 41-54View in Article -
- Carr E.L.
- Kelman A.
- Wu G.S.
- Gopaul R.
- Senkevitch E.
- Aghvanyan A.
- Turay A.M.
- Frauwirth K.A.
Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation.
J. Immunol. 2010; 185: 1037-1044View in Article -
- Sinclair L.V.
- Rolf J.
- Emslie E.
- Shi Y.B.
- Taylor P.M.
- Cantrell D.A.
Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation.
Nat. Immunol. 2013; 14: 500-508View in Article -
- Gerriets V.A.
- Rathmell J.C.
Metabolic pathways in T cell fate and function.
Trends Immunol. 2012; 33: 168-173View in Article -
- Michalek R.D.
- Gerriets V.A.
- Jacobs S.R.
- Macintyre A.N.
- MacIver N.J.
- Mason E.F.
- Sullivan S.A.
- Nichols A.G.
- Rathmell J.C.
Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets.
J. Immunol. 2011; 186: 3299-3303View in Article -
- Mascanfroni I.D.
- Takenaka M.C.
- Yeste A.
- Patel B.
- Wu Y.
- Kenison J.E.
- Siddiqui S.
- Basso A.S.
- Otterbein L.E.
- Pardoll D.M.
- Pan F.
- Priel A.
- Clish C.B.
- Robson S.C.
- Quintana F.J.
Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α.
Nat. Med. 2015; 21: 638-646View in Article -
- Doughty C.A.
- Bleiman B.F.
- Wagner D.J.
- Dufort F.J.
- Mataraza J.M.
- Roberts M.F.
- Chiles T.C.
Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth.
Blood. 2006; 107: 4458-4465View in Article -
- Dufort F.J.
- Bleiman B.F.
- Gumina M.R.
- Blair D.
- Wagner D.J.
- Roberts M.F.
- Abu-Amer Y.
- Chiles T.C.
Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism.
J. Immunol. 2007; 179: 4953-4957View in Article -
- Caro-Maldonado A.
- Wang R.
- Nichols A.G.
- Kuraoka M.
- Milasta S.
- Sun L.D.
- Gavin A.L.
- Abel E.D.
- Kelsoe G.
- Green D.R.
- Rathmell J.C.
Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells.
J. Immunol. 2014; 192: 3626-3636View in Article -
- Donnelly R.P.
- Loftus R.M.
- Keating S.E.
- Liou K.T.
- Biron C.A.
- Gardiner C.M.
- Finlay D.K.
mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function.
J. Immunol. 2014; 193: 4477-4484View in Article -
- Pollizzi K.N.
- Powell J.D.
Integrating canonical and metabolic signalling programmes in the regulation of T cell responses.
Nat. Rev. Immunol. 2014; 14: 435-446View in Article -
- Finlay D.K.
- Rosenzweig E.
- Sinclair L.V.
- Feijoo-Carnero C.
- Hukelmann J.L.
- Rolf J.
- Panteleyev A.A.
- Okkenhaug K.
- Cantrell D.A.
PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells.
J. Exp. Med. 2012; 209: 2441-2453View in Article -
- Oestreich K.J.
- Read K.A.
- Gilbertson S.E.
- Hough K.P.
- McDonald P.W.
- Krishnamoorthy V.
- Weinmann A.S.
Bcl-6 directly represses the gene program of the glycolysis pathway.
Nat. Immunol. 2014; 15: 957-964View in Article -
- Shi L.Z.
- Wang R.
- Huang G.
- Vogel P.
- Neale G.
- Green D.R.
- Chi H.
HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells.
J. Exp. Med. 2011; 208: 1367-1376View in Article -
- Tannahill G.M.
- Curtis A.M.
- Adamik J.
- Palsson-McDermott E.M.
- McGettrick A.F.
- Goel G.
- Frezza C.
- Bernard N.J.
- Kelly B.
- Foley N.H.
- Zheng L.
- Gardet A.
- Tong Z.
- Jany S.S.
- Corr S.C.
- Haneklaus M.
- Caffrey B.E.
- Pierce K.
- Walmsley S.
- Beasley F.C.
- Cummins E.
- Nizet V.
- Whyte M.
- Taylor C.T.
- Lin H.
- Masters S.L.
- Gottlieb E.
- Kelly V.P.
- Clish C.
- Auron P.E.
- Xavier R.J.
- O'Neill L.A.
Succinate is an inflammatory signal that induces IL-1β through HIF-1α.
Nature. 2013; 496: 238-242View in Article -
- Borregaard N.
- Herlin T.
Energy metabolism of human neutrophils during phagocytosis.
J. Clin. Invest. 1982; 70: 550-557View in Article -
- Fossati G.
- Moulding D.A.
- Spiller D.G.
- Moots R.J.
- White M.R.
- Edwards S.W.
The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis.
J. Immunol. 2003; 170: 1964-1972View in Article -
- Maianski N.A.
- Geissler J.
- Srinivasula S.M.
- Alnemri E.S.
- Roos D.
- Kuijpers T.W.
Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis.
Cell Death Differ. 2004; 11: 143-153View in Article -
- Newsholme P.
- Curi R.
- Gordon S.
- Newsholme E.A.
Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages.
Biochem. J. 1986; 239: 121-125View in Article -
- Krawczyk C.M.
- Holowka T.
- Sun J.
- Blagih J.
- Amiel E.
- DeBerardinis R.J.
- Cross J.R.
- Jung E.
- Thompson C.B.
- Jones R.G.
- Pearce E.J.
Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation.
Blood. 2010; 115: 4742-4749View in Article -
- Pearce E.J.
- Everts B.
Dendritic cell metabolism.
Nat. Rev. Immunol. 2015; 15: 18-29View in Article -
- Jha A.K.
- Huang S.C.
- Sergushichev A.
- Lampropoulou V.
- Ivanova Y.
- Loginicheva E.
- Chmielewski K.
- Stewart K.M.
- Ashall J.
- Everts B.
- Pearce E.J.
- Driggers E.M.
- Artyomov M.N.
Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization.
Immunity. 2015; 42: 419-430View in Article -
- Michelucci A.
- Cordes T.
- Ghelfi J.
- Pailot A.
- Reiling N.
- Goldmann O.
- Binz T.
- Wegner A.
- Tallam A.
- Rausell A.
- Buttini M.
- Linster C.L.
- Medina E.
- Balling R.
- Hiller K.
Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production.
Proc. Natl. Acad. Sci. U.S.A. 2013; 110: 7820-7825View in Article -
- Everts B.
- Amiel E.
- Huang S.C.
- Smith A.M.
- Chang C.H.
- Lam W.Y.
- Redmann V.
- Freitas T.C.
- Blagih J.
- van der Windt G.J.
- Artyomov M.N.
- Jones R.G.
- Pearce E.L.
- Pearce E.J.
TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation.
Nat. Immunol. 2014; 15: 323-332View in Article -
- Amiel E.
- Everts B.
- Freitas T.C.
- King I.L.
- Curtis J.D.
- Pearce E.L.
- Pearce E.J.
Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice.
J. Immunol. 2012; 189: 2151-2158View in Article -
- Amiel E.
- Everts B.
- Fritz D.
- Beauchamp S.
- Ge B.
- Pearce E.L.
- Pearce E.J.
Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function.
J. Immunol. 2014; 193: 2821-2830View in Article -
- Rodríguez-Espinosa O.
- Rojas-Espinosa O.
- Moreno-Altamirano M.M.
- López-Villegas E.O.
- Sánchez-García F.J.
Metabolic requirements for neutrophil extracellular traps formation.
Immunology. 2015; 145: 213-224View in Article -
- Azevedo E.P.
- Rochael N.C.
- Guimarães-Costa A.B.
- de Souza-Vieira T.S.
- Ganilho J.
- Saraiva E.M.
- Palhano F.L.
- Foguel D.
A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation.
J. Biol. Chem. 2015; 290: 22174-22183View in Article -
- McInturff A.M.
- Cody M.J.
- Elliott E.A.
- Glenn J.W.
- Rowley J.W.
- Rondina M.T.
- Yost C.C.
Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α.
Blood. 2012; 120: 3118-3125View in Article -
- Sumbayev V.V.
- Nicholas S.A.
- Streatfield C.L.
- Gibbs B.F.
Involvement of hypoxia-inducible factor-1 HiF(1α) in IgE-mediated primary human basophil responses.
Eur. J. Immunol. 2009; 39: 3511-3519View in Article -
- van der Windt G.J.
- O'Sullivan D.
- Everts B.
- Huang S.C.
- Buck M.D.
- Curtis J.D.
- Chang C.H.
- Smith A.M.
- Ai T.
- Faubert B.
- Jones R.G.
- Pearce E.J.
- Pearce E.L.
CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability.
Proc. Natl. Acad. Sci. U.S.A. 2013; 110: 14336-14341View in Article -
- Cui G.
- Staron M.M.
- Gray S.M.
- Ho P.C.
- Amezquita R.A.
- Wu J.
- Kaech S.M.
IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity.
Cell. 2015; 161: 750-761View in Article -
- O'Sullivan D.
- van der Windt G.J.
- Huang S.C.
- Curtis J.D.
- Chang C.H.
- Buck M.D.
- Qiu J.
- Smith A.M.
- Lam W.Y.
- DiPlato L.M.
- Hsu F.F.
- Birnbaum M.J.
- Pearce E.J.
- Pearce E.L.
Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development.
Immunity. 2014; 41: 75-88View in Article -
- Gubser P.M.
- Bantug G.R.
- Razik L.
- Fischer M.
- Dimeloe S.
- Hoenger G.
- Durovic B.
- Jauch A.
- Hess C.
Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch.
Nat. Immunol. 2013; 14: 1064-1072View in Article -
- MacIver N.J.
- Michalek R.D.
- Rathmell J.C.
Metabolic regulation of T lymphocytes.
Annu. Rev. Immunol. 2013; 31: 259-283View in Article -
- Mantovani A.
- Biswas S.K.
- Galdiero M.R.
- Sica A.
- Locati M.
Macrophage plasticity and polarization in tissue repair and remodelling.
J. Pathol. 2013; 229: 176-185View in Article -
- Gerriets V.A.
- Kishton R.J.
- Nichols A.G.
- Macintyre A.N.
- Inoue M.
- Ilkayeva O.
- Winter P.S.
- Liu X.
- Priyadharshini B.
- Slawinska M.E.
- Haeberli L.
- Huck C.
- Turka L.A.
- Wood K.C.
- Hale L.P.
- Smith P.A.
- Schneider M.A.
- MacIver N.J.
- Locasale J.W.
- Newgard C.B.
- Shinohara M.L.
- Rathmell J.C.
Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation.
J. Clin. Invest. 2015; 125: 194-207View in Article -
- Tan Z.
- Xie N.
- Cui H.
- Moellering D.R.
- Abraham E.
- Thannickal V.J.
- Liu G.
Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism.
J. Immunol. 2015; 194: 6082-6089View in Article -
- Vats D.
- Mukundan L.
- Odegaard J.I.
- Zhang L.
- Smith K.L.
- Morel C.R.
- Wagner R.A.
- Greaves D.R.
- Murray P.J.
- Chawla A.
Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation.
Cell Metab. 2006; 4: 13-24View in Article -
- Huang S.C.
- Everts B.
- Ivanova Y.
- O'Sullivan D.
- Nascimento M.
- Smith A.M.
- Beatty W.
- Love-Gregory L.
- Lam W.Y.
- O'Neill C.M.
- Yan C.
- Du H.
- Abumrad N.A.
- Urban Jr., J.F.
- Artyomov M.N.
- Pearce E.L.
- Pearce E.J.
Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages.
Nat. Immunol. 2014; 15: 846-855View in Article -
- Maciolek J.A.
- Pasternak J.A.
- Wilson H.L.
Metabolism of activated T lymphocytes.
Curr. Opin. Immunol. 2014; 27: 60-74View in Article -
- Peiris J.S.
- Hui K.P.
- Yen H.L.
Host response to influenza virus: protection versus immunopathology.
Curr. Opin. Immunol. 2010; 22: 475-481View in Article -
- Pearce E.L.
- Walsh M.C.
- Cejas P.J.
- Harms G.M.
- Shen H.
- Wang L.S.
- Jones R.G.
- Choi Y.
Enhancing CD8 T-cell memory by modulating fatty acid metabolism.
Nature. 2009; 460: 103-107View in Article -
- Sukumar M.
- Liu J.
- Ji Y.
- Subramanian M.
- Crompton J.G.
- Yu Z.
- Roychoudhuri R.
- Palmer D.C.
- Muranski P.
- Karoly E.D.
- Mohney R.P.
- Klebanoff C.A.
- Lal A.
- Finkel T.
- Restifo N.P.
- Gattinoni L.
Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function.
J. Clin. Invest. 2013; 123: 4479-4488View in Article -
- Six E.
- Lagresle-Peyrou C.
- Susini S.
- De Chappedelaine C.
- Sigrist N.
- Sadek H.
- Chouteau M.
- Cagnard N.
- Fontenay M.
- Hermine O.
- Chomienne C.
- Reynier P.
- Fischer A.
- André-Schmutz I.
- Gueguen N.
- Cavazzana M.
AK2 deficiency compromises the mitochondrial energy metabolism required for differentiation of human neutrophil and lymphoid lineages.
Cell Death Dis. 2015; 6: e1856View in Article -
- Rivadeneira D.B.
- Caino M.C.
- Seo J.H.
- Angelin A.
- Wallace D.C.
- Languino L.R.
- Altieri D.C.
Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion.
Sci. Signal. 2015; 8: ra80View in Article -
- Maryanovich M.
- Zaltsman Y.
- Ruggiero A.
- Goldman A.
- Shachnai L.
- Zaidman S.L.
- Porat Z.
- Golan K.
- Lapidot T.
- Gross A.
An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate.
Nat. Commun. 2015; 6: 7901View in Article -
- Leunda-Casi A.
- Genicot G.
- Donnay I.
- Pampfer S.
- De Hertogh R.
Increased cell death in mouse blastocysts exposed to high d-glucose in vitro: implications of an oxidative stress and alterations in glucose metabolism.
Diabetologia. 2002; 45: 571-579View in Article -
- MacFarlane M.
- Robinson G.L.
- Cain K.
Glucose—a sweet way to die: metabolic switching modulates tumor cell death.
Cell Cycle. 2012; 11: 3919-3925View in Article -
- Robinson G.L.
- Dinsdale D.
- Macfarlane M.
- Cain K.
Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL.
Oncogene. 2012; 31: 4996-5006View in Article -
- Shim H.
- Chun Y.S.
- Lewis B.C.
- Dang C.V.
A unique glucose-dependent apoptotic pathway induced by c-Myc.
Proc. Natl. Acad. Sci. U.S.A. 1998; 95: 1511-1516View in Article -
- Vander Heiden M.G.
- Plas D.R.
- Rathmell J.C.
- Fox C.J.
- Harris M.H.
- Thompson C.B.
Growth factors can influence cell growth and survival through effects on glucose metabolism.
Mol. Cell. Biol. 2001; 21: 5899-5912View in Article -
- Lenardo M.
- Chan K.M.
- Hornung F.
- McFarland H.
- Siegel R.
- Wang J.
- Zheng L.
Mature T lymphocyte apoptosis: immune regulation in a dynamic and unpredictable antigenic environment.
Annu. Rev. Immunol. 1999; 17: 221-253View in Article -
- Chang C.H.
- Curtis J.D.
- Maggi Jr., L.B.
- Faubert B.
- Villarino A.V.
- O'Sullivan D.
- Huang S.C.
- van der Windt G.J.
- Blagih J.
- Qiu J.
- Weber J.D.
- Pearce E.J.
- Jones R.G.
- Pearce E.L.
Posttranscriptional control of T cell effector function by aerobic glycolysis.
Cell. 2013; 153: 1239-1251View in Article -
- Zang W.Q.
- Fieno A.M.
- Grant R.A.
- Yen T.S.
Identification of glyceraldehyde-3-phosphate dehydrogenase as a cellular protein that binds to the hepatitis B virus posttranscriptional regulatory element.
Virology. 1998; 248: 46-52View in Article -
- Schultz D.E.
- Hardin C.C.
- Lemon S.M.
Specific interaction of glyceraldehyde 3-phosphate dehydrogenase with the 5′-nontranslated RNA of hepatitis A virus.
J. Biol. Chem. 1996; 271: 14134-14142View in Article -
- Nagy E.
- Rigby W.F.
Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD+-binding region (Rossmann fold).
J. Biol. Chem. 1995; 270: 2755-2763View in Article -
- De B.P.
- Gupta S.
- Zhao H.
- Drazba J.A.
- Banerjee A.K.
Specific interaction in vitro and in vivo of glyceraldehyde-3-phosphate dehydrogenase and LA protein with cis-acting RNAs of human parainfluenza virus type 3.
J. Biol. Chem. 1996; 271: 24728-24735View in Article -
- Mukhopadhyay R.
- Jia J.
- Arif A.
- Ray P.S.
- Fox P.L.
The GAIT system: a gatekeeper of inflammatory gene expression.
Trends Biochem. Sci. 2009; 34: 324-331View in Article -
- Castello A.
- Fischer B.
- Eichelbaum K.
- Horos R.
- Beckmann B.M.
- Strein C.
- Davey N.E.
- Humphreys D.T.
- Preiss T.
- Steinmetz L.M.
- Krijgsveld J.
- Hentze M.W.
Insights into RNA biology from an atlas of mammalian mRNA-binding proteins.
Cell. 2012; 149: 1393-1406View in Article -
- Noman M.Z.
- Desantis G.
- Janji B.
- Hasmim M.
- Karray S.
- Dessen P.
- Bronte V.
- Chouaib S.
PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation.
J. Exp. Med. 2014; 211: 781-790View in Article -
- Kimura A.
- Naka T.
- Nohara K.
- Fujii-Kuriyama Y.
- Kishimoto T.
Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells.
Proc. Natl. Acad. Sci. U.S.A. 2008; 105: 9721-9726View in Article -
- Veldhoen M.
- Hirota K.
- Westendorf A.M.
- Buer J.
- Dumoutier L.
- Renauld J.C.
- Stockinger B.
The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins.
Nature. 2008; 453: 106-109View in Article -
- Quintana F.J.
- Basso A.S.
- Iglesias A.H.
- Korn T.
- Farez M.F.
- Bettelli E.
- Caccamo M.
- Oukka M.
- Weiner H.L.
Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor.
Nature. 2008; 453: 65-71View in Article -
- Apetoh L.
- Quintana F.J.
- Pot C.
- Joller N.
- Xiao S.
- Kumar D.
- Burns E.J.
- Sherr D.H.
- Weiner H.L.
- Kuchroo V.K.
The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27.
Nat. Immunol. 2010; 11: 854-861View in Article -
- Kidani Y.
- Elsaesser H.
- Hock M.B.
- Vergnes L.
- Williams K.J.
- Argus J.P.
- Marbois B.N.
- Komisopoulou E.
- Wilson E.B.
- Osborne T.F.
- Graeber T.G.
- Reue K.
- Brooks D.G.
- Bensinger S.J.
Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity.
Nat. Immunol. 2013; 14: 489-499View in Article -
- Cui G.
- Qin X.
- Wu L.
- Zhang Y.
- Sheng X.
- Yu Q.
- Sheng H.
- Xi B.
- Zhang J.Z.
- Zang Y.Q.
Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation.
J. Clin. Invest. 2011; 121: 658-670View in Article -
- Im S.S.
- Yousef L.
- Blaschitz C.
- Liu J.Z.
- Edwards R.A.
- Young S.G.
- Raffatellu M.
- Osborne T.F.
Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a.
Cell Metab. 2011; 13: 540-549View in Article -
- Sena L.A.
- Li S.
- Jairaman A.
- Prakriya M.
- Ezponda T.
- Hildeman D.A.
- Wang C.R.
- Schumacker P.T.
- Licht J.D.
- Perlman H.
- Bryce P.J.
- Chandel N.S.
Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling.
Immunity. 2013; 38: 225-236View in Article -
- Selak M.A.
- Armour S.M.
- MacKenzie E.D.
- Boulahbel H.
- Watson D.G.
- Mansfield K.D.
- Pan Y.
- Simon M.C.
- Thompson C.B.
- Gottlieb E.
Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase.
Cancer Cell. 2005; 7: 77-85View in Article -
- Koivunen P.
- Hirsilä M.
- Remes A.M.
- Hassinen I.E.
- Kivirikko K.I.
- Myllyharju J.
Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF.
J. Biol. Chem. 2007; 282: 4524-4532View in Article -
- Xiao M.
- Yang H.
- Xu W.
- Ma S.
- Lin H.
- Zhu H.
- Liu L.
- Liu Y.
- Yang C.
- Xu Y.
- Zhao S.
- Ye D.
- Xiong Y.
- Guan K.L.
Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors.
Genes Dev. 2012; 26: 1326-1338View in Article -
- Smith E.H.
- Janknecht R.
- Maher 3rd, L.J.
Succinate inhibition of α-ketoglutarate-dependent enzymes in a yeast model of paraganglioma.
Hum. Mol. Genet. 2007; 16: 3136-3148View in Article -
- Mills E.
- O'Neill L.A.
Succinate: a metabolic signal in inflammation.
Trends Cell Biol. 2014; 24: 313-320View in Article -
- Rubic T.
- Lametschwandtner G.
- Jost S.
- Hinteregger S.
- Kund J.
- Carballido-Perrig N.
- Schwärzler C.
- Junt T.
- Voshol H.
- Meingassner J.G.
- Mao X.
- Werner G.
- Rot A.
- Carballido J.M.
Triggering the succinate receptor GPR91 on dendritic cells enhances immunity.
Nat. Immunol. 2008; 9: 1261-1269View in Article -
- Infantino V.
- Convertini P.
- Cucci L.
- Panaro M.A.
- Di Noia M.A.
- Calvello R.
- Palmieri F.
- Iacobazzi V.
The mitochondrial citrate carrier: a new player in inflammation.
Biochem. J. 2011; 438: 433-436View in Article -
- Ichiyama K.
- Chen T.
- Wang X.
- Yan X.
- Kim B.S.
- Tanaka S.
- Ndiaye-Lobry D.
- Deng Y.
- Zou Y.
- Zheng P.
- Tian Q.
- Aifantis I.
- Wei L.
- Dong C.
The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells.
Immunity. 2015; 42: 613-626View in Article -
- De Santa F.
- Narang V.
- Yap Z.H.
- Tusi B.K.
- Burgold T.
- Austenaa L.
- Bucci G.
- Caganova M.
- Notarbartolo S.
- Casola S.
- Testa G.
- Sung W.K.
- Wei C.L.
- Natoli G.
Jmjd3 contributes to the control of gene expression in LPS-activated macrophages.
EMBO J. 2009; 28: 3341-3352View in Article -
- Cai L.
- Sutter B.M.
- Li B.
- Tu B.P.
Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes.
Mol. Cell. 2011; 42: 426-437View in Article -
- Cheng S.C.
- Quintin J.
- Cramer R.A.
- Shepardson K.M.
- Saeed S.
- Kumar V.
- Giamarellos-Bourboulis E.J.
- Martens J.H.
- Rao N.A.
- Aghajanirefah A.
- Manjeri G.R.
- Li Y.
- Ifrim D.C.
- Arts R.J.
- van der Veer B.M.
- Deen P.M.
- Logie C.
- O'Neill L.A.
- Willems P.
- van de Veerdonk F.L.
- van der Meer J.W.
- Ng A.
- Joosten L.A.
- Wijmenga C.
- Stunnenberg H.G.
- Xavier R.J.
- Netea M.G.
mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity.
Science. 2014; 345: 1250684View in Article -
- Lin S.J.
- Ford E.
- Haigis M.
- Liszt G.
- Guarente L.
Calorie restriction extends yeast life span by lowering the level of NADH.
Genes Dev. 2004; 18: 12-16View in Article -
- Kwon H.S.
- Lim H.W.
- Wu J.
- Schnölzer M.
- Verdin E.
- Ott M.
Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells.
J. Immunol. 2012; 188: 2712-2721View in Article -
- Beier U.H.
- Wang L.
- Bhatti T.R.
- Liu Y.
- Han R.
- Ge G.
- Hancock W.W.
Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival.
Mol. Cell. Biol. 2011; 31: 1022-1029View in Article -
- van Loosdregt J.
- Vercoulen Y.
- Guichelaar T.
- Gent Y.Y.
- Beekman J.M.
- van Beekum O.
- Brenkman A.B.
- Hijnen D.J.
- Mutis T.
- Kalkhoven E.
- Prakken B.J.
- Coffer P.J.
Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization.
Blood. 2010; 115: 965-974View in Article -
- Lim H.W.
- Kang S.G.
- Ryu J.K.
- Schilling B.
- Fei M.
- Lee I.S.
- Kehasse A.
- Shirakawa K.
- Yokoyama M.
- Schnölzer M.
- Kasler H.G.
- Kwon H.S.
- Gibson B.W.
- Sato H.
- Akassoglou K.
- Xiao C.
- Littman D.R.
- Ott M.
- Verdin E.
SIRT1 deacetylates RORγt and enhances Th17 cell generation.
J. Exp. Med. 2015; 212: 607-617View in Article -
- Yeung F.
- Hoberg J.E.
- Ramsey C.S.
- Keller M.D.
- Jones D.R.
- Frye R.A.
- Mayo M.W.
Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase.
EMBO J. 2004; 23: 2369-2380View in Article -
- Zhang J.
- Lee S.M.
- Shannon S.
- Gao B.
- Chen W.
- Chen A.
- Divekar R.
- McBurney M.W.
- Braley-Mullen H.
- Zaghouani H.
- Fang D.
The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice.
J. Clin. Invest. 2009; 119: 3048-3058View in Article -
- Patsoukis N.
- Bardhan K.
- Chatterjee P.
- Sari D.
- Liu B.
- Bell L.N.
- Karoly E.D.
- Freeman G.J.
- Petkova V.
- Seth P.
- Li L.
- Boussiotis V.A.
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation.
Nat. Commun. 2015; 6: 6692View in Article



