29.6: Metabolic consequences of obesity and type 2 diabetes- Balancing genes and environment for personalized care
- Page ID
- 53879
Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care
Nicolas J. Pillon, Ruth J.F. Loos, Sally M. Marshall, Juleen R. Zierath,. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell, Volume 184, Issue 6, 2021, Pages 1530-1544, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2021.02.012.
Under a Creative Commons license. Attribution 4.0 International (CC BY 4.0)
Summary
The prevalence of type 2 diabetes and obesity has risen dramatically for decades and is expected to rise further, secondary to the growing aging, sedentary population. The strain on global health care is projected to be colossal. This review explores the latest work and emerging ideas related to genetic and environmental factors influencing metabolism. Translational research and clinical applications, including the impact of the COVID-19 pandemic, are highlighted. Looking forward, strategies to personalize all aspects of prevention, management and care are necessary to improve health outcomes and reduce the impact of these metabolic diseases.
Introduction
The COVID-19 pandemic has brought the deleterious health consequences of obesity and type 2 diabetes into sharp focus. Individuals with type 2 diabetes and/or obesity are more likely to have severe disease and to die than are individuals without diabetes (Barron et al., 2020). Fasting glucose level at the time of hospital admission predicts 28-day mortality even in those without a previous diagnosis of diabetes (Wang et al., 2020a). Glycemic control and body mass index along with older age, male sex, socio-economic deprivation, non-white ethnicity, and pre-existing renal and cardiovascular disease all independently increase mortality (Holman et al., 2020). COVID-19 is also a timely reminder that diabetes is not merely a state of glucose dysregulation but a multi-faceted syndrome driven by many medical and social risk factors and associated with pathophysiological changes throughout the body.
The World Health Organization estimates that worldwide, 422 million people have diabetes, the majority living in low- and middle-income countries, and most having type 2 diabetes (who.int/health-topics/diabetes). The prevalence has risen dramatically for decades, as the population ages and becomes less active and more overweight (GBD 2019 Risk Factors Collaborators, 2020). Early detection is vital, particularly as long-term complications, such as referable diabetic retinopathy, may be present at diagnosis of type 2 diabetes (Kohner et al., 1998). Many developed countries have systematic screening programs of individuals deemed to be at high risk (American Diabetes Association, 2020). However, there is disagreement as to how to define “high risk” and how to screen (oral glucose tolerance test, fasting glucose or glycated hemoglobin, HbA1c). Glucose-based tests and HbA1c each identify slightly different populations. We do not know if these differences in diagnoses lead to important clinically different outcomes or if they signal slightly different pathological metabolic forms of glucose dysregulation (American Diabetes Association, 2020).
The WHO defines overweight and obesity as body mass indexes (BMI) 3 25 and 30 kg/m2, respectively and estimated that 1.9 billion adults were overweight and 650 million obese in 2016 (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Obesity is now regarded as a chronic, progressive disease with remissions and relapses (Bray et al., 2017) and an important driver of the development of diabetes and many of its associated features (GBD 2019 Risk Factors Collaborators, 2020). The deleterious effects of obesity and type 2 diabetes are seen in most, if not all, tissues in the body, with consequences resulting in significantly increased premature morbidity and mortality (GBD 2019 Risk Factors Collaborators, 2020). Social and cultural factors are also extremely important in the development, management, and clinical outcomes of obesity and type 2 diabetes.
Despite advances in diabetes care over the recent decades, there remain vast challenges: developing an improved understanding of the heterogeneity of obesity and diabetes, how best to assess risk, to screen, to select individualized treatments and vitally how to engage the relevant populations in these programs. This review explores the genetic and metabolic aspects of diabetes and obesity (Figure 1) and discusses some of the latest work and emerging ideas related to basic biological mechanisms, translational research, and clinical applications.
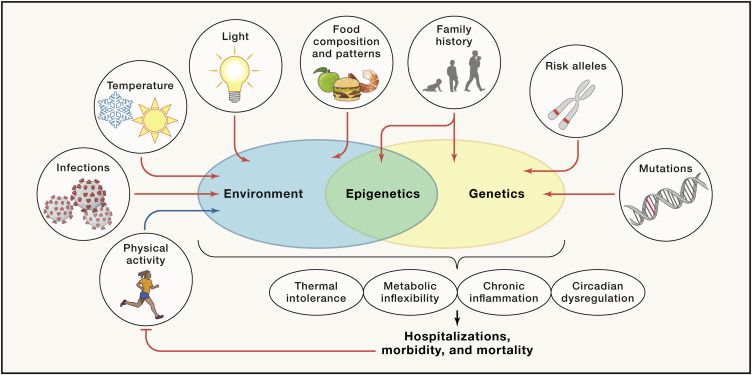
Figure 1. Gene-environment interactions regulating disease risk of obesity and type 2 diabetes
Individual genetic predispositions and environmental factors interact to promote or impair molecular processes, such as circadian regulation, thermal tolerance, and/or chronic inflammation. Accumulation of genetic and environment risk factors eventually leads to the development of complications, reducing both healthspan and lifespan.
Genetics and metabolism
The current obesogenic environment, favoring high-calorie foods and physical inactivity, is a major driver of the growing obesity and diabetes epidemic. However, not everyone exposed to this environment gains weight or develops type 2 diabetes. The way people respond to environmental factors is, at least in part, determined by their genetic predisposition to obesity and type 2 diabetes. Traditionally, the genetic contribution has been quantified by the heritability, which is a population-level estimate of how much of the variation in disease susceptibility is attributable to genetic variation. For obesity and type 2 diabetes, the heritability has been estimated to be moderate-to-high, ranging between 30% and 70% (Elks et al., 2012; Willemsen et al., 2015). The search for contributing genes started in the 1990’s with early success largely confined to monogenic forms of obesity and diabetes. Mutations that segregate in families or occur de novo were found to cause major disruptions in the function of genes in which they are located, providing the first insights in the pathophysiology of body-weight regulation and glucose metabolism (Hattersley and Patel, 2017; van der Klaauw and Farooqi, 2015). The search for genetic variants that contribute to common forms of obesity and diabetes began slowly with candidate gene and genome-wide linkage studies. However, the advent of genome-wide association studies (GWASs) in the mid-2000’s accelerated the pace of gene discovery.
GWASs have identified thousands of genetic loci that are robustly associated with complex diseases and traits, including 700 for obesity (Yengo et al., 2018) and at least 400 for type 2 diabetes (Mahajan et al., 2018). From the earliest GWAS, tissue enrichment and pathway analyses for BMI-associated loci have suggested that the central nervous system plays a key role in body weight regulation (Locke et al., 2015). Loci associated with type 2 diabetes act predominantly through the perturbation of insulin secretion, pointing to the importance of beta cell function or mass, whereas few loci affect insulin resistance through an effect on body weight or fat distribution (Barroso and McCarthy, 2019).
Despite the success of GWASs, pinpointing the causal gene(s) and variant(s) within each locus remains an ongoing challenge. So far, about 20% of loci associated with type 2 diabetes and a handful of loci associated with obesity have been mapped to the most likely causal variant, whereas the underlying biology of hundreds of additional loci remain to be elucidated (Larder et al., 2017; Mahajan et al., 2018; Rathjen et al., 2017). However, with increasing availability of high-throughput genome-scale technologies for mapping regulatory elements, comprehensive multi-omics databases, advanced computational tools, and the latest genetic engineering and molecular phenotyping approaches, we are poised to accelerate the translation of GWAS loci into meaningful biology in the years ahead.
With GWASs, genetic susceptibility to disease can be assessed using polygenic scores. A polygenic score represents an individual’s overall genetic susceptibility to disease and is calculated by summing the number of disease-increasing alleles that were inherited from either parent, weighted by each variant’s effect size observed in a GWAS. Even though each locus has a small effect on disease risk and explains only a fraction of the variation in disease susceptibility, when aggregated in a polygenic score, their contribution can be substantial. Polygenic scores are normally distributed, with most individuals having an average score, and thus an average genetic susceptibility, whereas individuals at the extremes of the distribution have a (very) high or low genetic risk of disease. For example, in the UK Biobank, the average BMI of individuals with a high polygenic score (top decile) is 2.9 kg/m2 (equivalent to 8 kg in body weight) higher and their odds of severe obesity (BMI 3 40 kg/m2) is 4.2-fold higher, compared to those with a lower polygenic score (bottom 9 deciles) (Khera et al., 2019). Similarly, individuals with a very high polygenic score (top 5%) for type 2 diabetes have a 2.75-fold increased risk of disease compared to the remainder of the population (Udler et al., 2019).
These observations have fueled expectations that genotype information, including polygenic scores, can soon be used in clinical care for early diagnosis of high-risk individuals, to tailor prevention and treatments strategies, and to improve disease prognostics. In fact, many online direct-to-consumer genomic companies are already informing customers about their risks and predispositions for a range of common diseases and traits based solely on genetic profiling, including for obesity and type 2 diabetes (Figure 2). However, even though the genetic associations observed in GWASs are robust, their ability to predict who will be at a high risk of obesity or type 2 diabetes is still low-to-moderate, and not ready for use in clinical settings (Udler et al., 2019). For example, a recent polygenic score applied to individuals of European ancestry of the UK Biobank explains only 8%–9% of the variation in BMI and is a weak predictor of obesity, with an area under the receiver operating characteristic curve (AUCROC) of 0.64 (Khera et al., 2019). Findings are similar for polygenic scores for type 2 diabetes, with AUCROC of 0.64–0.66 (Udler et al., 2019). The predictive ability of polygenic scores are expected to improve as GWASs increase in sample size and the per-variant effect estimates become more precise, and as algorithms to aggregate millions of genetic variants across the genome improve. Nevertheless, given the importance of socio-demographic, lifestyle and clinical risk factors in the etiology of obesity and type 2 diabetes, it is unlikely that a polygenic score on its own will ever be able to accurately predict obesity or type 2 diabetes. More comprehensive approaches that include a broad spectrum of genetic, demographic, environmental, clinical, and possibly also molecular markers are needed to accurately predict who is at risk of gaining weight and/or developing type 2 diabetes.
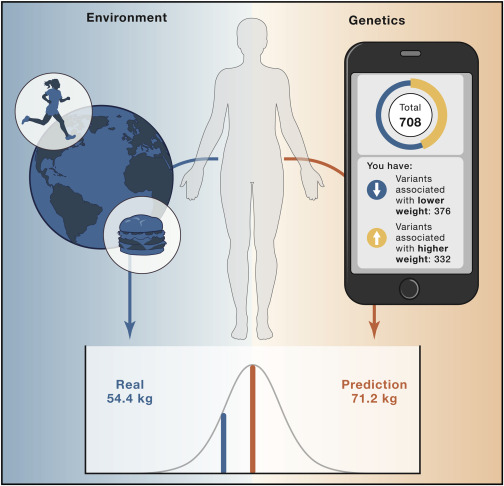
Figure 2. Genetic prediction of body weight—context matters
A 49-year old woman, who eats a balanced diet, runs 6 miles/day, and commutes by bike to work, does a direct-to-consumer genetic test with one of the many online personalized genomics companies. The company claims to provide genetic insight into her health to make it easier for her to take action. She provides a saliva sample and completes numerous questionnaires on her physical and mental health, family medical history, and more. Her reported results show that, based on the genetic variants tested, she carries 376 weight-lowering variants and 332 weight-increasing variants, predisposing her to weigh about “average” or 157 lbs (71.2 kg, based on the company’s customers’ weight of the same age, height, and sex). However, the woman’s real weight was 120 lbs (54.4 kg). A likely reason for why this genetic test overpredicted the woman’s weight by 30% is because her lifestyle—even though information was shared in detail—was not appropriately incorporated in the prediction models.
The vast amount of new genetic information generated by GWASs is being used in sub-typing disease at a population level. Obesity and type 2 diabetes are highly heterogeneous diseases, and the diagnosis of these metabolic diseases is unrefined, based on a single marker (BMI 3 30 kg/m2 and hyperglycemia, respectively). Consequently, individuals with the same diagnosis may differ considerably in disease pathogenesis, clinical presentation, disease course and response to treatments. Subtypes of obesity and type 2 diabetes have been typically based on phenotypic differences and similarities. As the number of GWAS-identified loci continues to increase, subtyping of obesity and type 2 diabetes based on genetic information has become possible. In a recent study, 141 variants previously identified for diabetes and diabetes-related traits were clustered in five groups, based on their association with more than 75 traits (Udler et al., 2018). Variants with a similar association profile cluster in the same group, and the group-specific association profile can inform about the mechanisms underlying a given subtype of type 2 diabetes. For example, two of the five groups identified for diabetes-related traits represent reduced beta-cell function, of which one cluster is characterized by high and the other by low proinsulin levels. The three other groups of variants show features of insulin resistance, of which one group represents obesity-mediated insulin resistance, a second group represents abnormal body fat distribution (“lipodystrophy-like”), and a third group represents disrupted liver lipid metabolism. Genetic risk scores based on variants in each cluster are associated with distinct clinical outcomes (Udler et al., 2018). Further for obesity, genotype information has been used to identify individuals who are predisposed to increased adiposity and, concomitantly, are protected from cardiometabolic outcomes (representing the so-called metabolically healthy obesity phenotype) (Ji et al., 2019). Subtyping of heterogenous diseases, like obesity and type 2 diabetes, is key to precision medicine. Indeed, these more homogeneous subgroups are characterized by distinct underlying biological mechanisms, such that diagnosis and prognosis will be more precise and optimization of treatment more efficient (Chung et al., 2020). As GWASs continue to identify more loci, additional and possibly better-defined clusters may be identified to more accurately represent the heterogenous group of individuals with obesity and type 2 diabetes.
As more GWAS loci are being discovered, Mendelian Randomization (MR) becomes an increasingly powerful approach to determine causality between an exposure (e.g., health-related behaviors, biomarkers [e.g., lipid levels, metabolites]) and an outcome (e.g., obesity, type 2 diabetes). Genetic variants that are robustly associated with the exposure are used to randomize a population in individuals with high exposure (i.e., carriers of the risk alleles) and those with low exposure (i.e., carriers of non-risk alleles). If the same genetic variants also associate with the disease outcome, through their association with the exposure, then causality between exposure and disease is inferred. For example, a large-scale MR study examined the causal role of a wide range of possible risk factors for type 2 diabetes, mostly confirming established risk factors, but also revealing new ones (e.g., insomnia) (Yuan and Larsson, 2020). As more GWAS data becomes available for a range of multi-omics biomarkers, MR analyses may reveal novel disease-causing biomarkers, broadening insights in the pathogenesis of obesity and type 2 diabetes.
Epigenetic impact on metabolism
Beyond genetic risk, the genes we inherit and the environmental factors we are exposed to can interact synergistically to modify our physiology and risk for obesity and type 2 diabetes through epigenetic modifications (Figure 3). Epigenetic modifications are biochemical processes that influence gene activity and expression, and ultimately modify cellular and whole-body physiology, without altering the DNA sequence of an organism’s genome (Barrès and Zierath, 2016). Mechanistically, epigenetic modifications can arise from chemical alterations of nucleosides in the DNA molecule itself by methylation or hydroxymethylation, alterations in chromatin structure or post-translational modifications of histones (i.e., methylation, phosphorylation, acetylation, ubiquitylation, and sumoylation) or RNA-associated gene silencing (Bošković and Rando, 2018). Although epigenetic modifications are generally thought to be fixed during development and maintained over an organism’s lifetime, there is some degree of plasticity in the epigenome, which engenders organismal adaptation to rapid environmental changes.
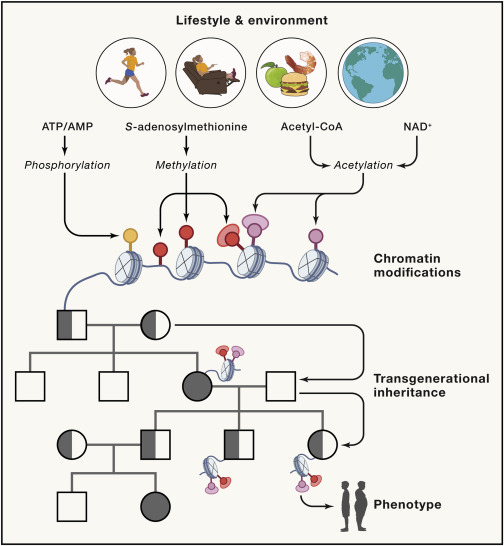
Figure 3. Epigenetic modifications in response to environmental factors lead to transgenerational effects on the phenotypes of offspring
Diet and exercise influence the cellular availability of nutrients impacting methylation, acetylation and phosphorylation of chromatin. Paternal or maternal environmental exposure can therefore influence metabolism and manifest obesity- or type 2 diabetes-related traits in the offspring through transgenerational epigenetic inheritance.
Alterations in nutritional status, food supply, physical activity/exercise, thermal stress, toxins, or other environmental insults can trigger epigenetic modifications and lead to genomic changes in somatic cells within an individual that directly disrupt metabolic homeostasis (Barrès and Zierath, 2016; Bošković and Rando, 2018). These same factors may also modify the physiology of an organism by transgenerational epigenetic inheritance, whereby paternal or maternal environmental exposure can influence metabolism and manifest obesity- or type 2 diabetes-related traits in the offspring. Prenatal undernutrition affects glucose tolerance and risk of diabetes in the offspring, as demonstrated by epidemiological studies of several famines over the past century (Li et al., 2010; Ravelli et al., 1998). In rodents, paternal and maternal diet and exercise influence metabolic and cardiovascular outcomes in offspring over several generations (de Castro Barbosa et al., 2015; Murashov et al., 2016; Stanford et al., 2015). Thus, nutritional status in utero during fetal development may affect the epigenome for several generations, but the molecular transducers remain to be clarified. Additionally, food restriction during childhood, at different growth phases around puberty, also leads to epigenetic changes that influence the risk of cardiovascular and metabolic disease of offspring over several generations (Kaati et al., 2002). Accordingly, epigenetic factors passed on by the gametes may contribute to the global increase in obesity and type 2 diabetes. Thus, an area of emerging interest is the influence of the environment on epigenetic mechanisms, and how this modifies metabolic disease risk.
A variety of dietary agents, as well as micronutrients and metabolites synthesized de novo, can serve as substrates or co-factors to influence the epigenome and potentially affect metabolic disease risk in humans, in part by affecting genomic plasticity (Tiffon, 2018). One-carbon metabolism encompasses folate and methionine cycles, which transfer one-carbon moieties and methyl groups for nucleotide synthesis, methylation reactions and reductive metabolism (Newman and Maddocks, 2017). Metabolites including acetyl-coA, AMP, NAD+, and S-adenosylmethionine are required for histone modifications (acetylation, phosphorylation) and methylation of DNA and histones. The extent to which nutritional factors, metabolites, and other co-factors directly modify the epigenome within a generation remains to be fully substantiated in humans.
While it is important to stress that type 2 diabetes and obesity are complex multi-factorial, progressive metabolic diseases with diverse etiology, and not simply “lifestyle disorders,” diet and exercise regimes can prevent or delay disease progression. Changes in the concentration of cellular metabolites, nucleotides, or calcium levels in skeletal muscle in response to acute exercise alter DNA methylation or histone modifications and influence gene expression through epigenetic mechanisms (Barrès and Zierath, 2016). In humans, acute exercise alters DNA methylation of the promoters of genes involved in metabolic regulation in skeletal muscle (Barrès et al., 2012; Nitert et al., 2012). Epigenetic modifications have also been observed in skeletal muscle and adipose tissue in humans with obesity and weight loss (Barres et al., 2013; Multhaup et al., 2015). Thus, the impact of environmental exposures and epigenetic influences on the risk for metabolic diseases throughout the lifespan is an important aspect of biology to unravel.
Circadian control of metabolism
An evolutionarily conserved mechanism by which environmental factors can impact whole-body physiology is through internal biological clocks and the control of circadian rhythms (Young, 2018). Circadian rhythms are driven by cell-autonomous intrinsic clocks that anticipate day/night cycles in order to optimize the physiology and behavior of organisms. Circadian programs are regulated at both the central and peripheral level with the master clock, located in the suprachiasmatic nucleus region of the hypothalamus, acting as conductor to synchronize and direct peripheral oscillators (Young, 2018). Synchronization of these intrinsic circadian clocks can be achieved in response to photic and non-photic zeitgebers (time-givers). The most powerful zeitgeber is light, which synchronizes the central clock. In addition to receiving cues from the central clock, peripheral clocks are synchronized by external zeitgebers, including food intake, temperature, energetic stressors, and drive the expression of a broad network of genes, many of which are involved in metabolic homeostasis (Gabriel and Zierath, 2019). The precise mechanism by which circadian clocks coordinate whole-body homeostatic processes is an area of emerging interest given the importance of external zeitgebers and the regulation of gene programs controlling metabolism and development.
One mechanism by which the circadian machinery influences metabolism is through the diurnal patterns of hormone secretion (Gamble et al., 2014). Endocrine organs release a variety of hormones in response to diverse environmental factors including diurnal cycles of light/dark, fasting/feeding, and temperature changes. For example, there are diurnal or circadian patterns of secretion of cortisol, growth hormone, prolactin, thyroid hormone, gonadal steroids, and melatonin related to sleep/wake cycles, whereas metabolic hormones including insulin, leptin, ghrelin, and glucagon vary in response to nutritional cues related to fasting/feeding cycles (Gamble et al., 2014). Many of these hormones including insulin, insulin-like growth factor 1, and glucocorticoids can act as zeitgebers to reset or fine tune the clock (Balsalobre et al., 2000; Crosby et al., 2019). Thus, an intimate relationship between circadian clocks and endocrine systems exists. This relationship is clinically relevant since disruption of the circadian clock is linked to metabolic disease.
In humans, long duration of shift work is associated with an increased risk of type 2 diabetes, which is only partly explained by lifestyle factors and BMI (Vimalananda et al., 2015). Epidemiological studies show that disruption of the sleep/wake cycle through extended periods of rotating night shift work is associated with obesity and increased risk of type 2 diabetes (Lin et al., 2009; Pan et al., 2011). Chronic jet lag in mouse models disrupts exergy homeostasis and leptin signaling and leads to circadian dysfunction-induced obesity (Kettner et al., 2015). Similarly, a population-based cohort study indicates that social jet lag, defined as the discrepancy between circadian and social clocks, is associated with increased risk of metabolic syndrome and diabetes/prediabetes (Koopman et al., 2017). Thus, chronobiology has implications for obesity and type 2 diabetes pathogenesis.
A basic paradigm of circadian regulation of metabolism is that oscillations of gene expression generate daily rhythms in cellular metabolism (Kim and Lazar, 2020). At the molecular level, circadian rhythms are generated by a cell autonomous and self-sustained transcriptional auto-regulatory feedback loop that is composed of transcriptional activators and their target genes, which rhythmically accumulate and form a repressor complex to inhibit transcriptional activity (Figure 4). Energy, nutrient, and oxygen sensors interact with the circadian clock machinery to control metabolic outputs including mitochondrial function, substrate utilization, insulin sensitivity, and glycemic control (Lamia et al., 2009; Peek et al., 2017; Sato et al., 2019). These sensors monitor oxygen availability and energy stress via hypoxia-inducible factor-1 alpha (HIF1α) and AMP-activated protein kinase (AMPK), respectively. Cells also integrate signals from nutrients and growth factors via mammalian target of rapamycin (mTOR). These energetic sensors not only exhibit circadian rhythmicity, but also regulate components of the core clock machinery through epigenetic modifications, mainly involving histone modifications (Kim and Lazar, 2020). Thus, cross-talk exists between the circadian clock and epigenetic factors that influence the genomic plasticity of organs controlling metabolic homeostasis. In rodents, dysregulation of the intrinsic molecular clock in a variety of tissues leads to obesity, insulin resistance, and altered glucose homeostasis (Rudic et al., 2004; Turek et al., 2005). Nevertheless, the mechanisms underlying disrupted circadian rhythmicity in people with type 2 diabetes are unknown. There is potential to coordinate behavioral changes with the body’s daily rhythm to improve metabolic homeostasis. Timing of exercise training bouts or meals and distribution of calories throughout the day may lead to improved outcomes for people with obesity or type 2 diabetes (Lundell et al., 2020; Savikj et al., 2019).
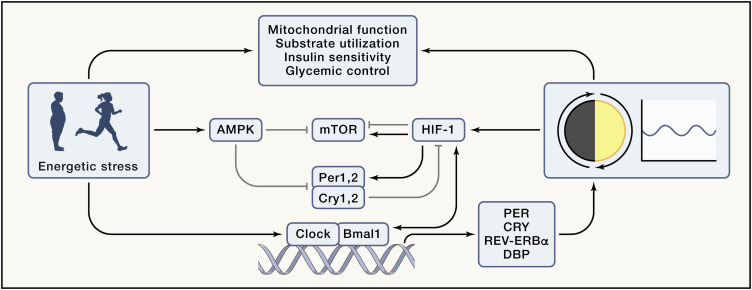
Figure 4. Circadian control and influence of energy sensing pathways
Mitochondrial function, substrate utilization, insulin sensitivity, and glycemic control exhibit diurnal rhythms that are influenced by a variety of factors including energetic stressors such as diet, exercise, and metabolic disease, as well as intrinsic clocks. The molecular circadian clock is composed of transcriptional activators, circadian locomotor output cycles kaput (CLOCK), and brain and muscle ARNTL-like protein 1 (BMAL1), and their target genes period (PER), cryptochrome (CRY), NR1D1 (which encodes REV-ERBα) and DBP, which rhythmically accumulate and form a repressor complex that interacts with CLOCK and BMAL1 to inhibit transcriptional activity. Energetic stressors influence the circadian program and metabolism. AMPK-mediated phosphorylation of CRY and PER promotes their destabilization and degradation, while mTOR activation induces CRY1 expression. PER2 inhibits mTOR complex activity via the tuberous sclerosis complex 1. HIF1α regulates PER2 transcription and interacts with BMAL1 at the chromatin level. CRY1 reduces HIF1α half-life by interacting with its basic-helix-loop-helix domain.
Impact of energetic stressors on the control of metabolism
Obesity, diabetes, exercise, and food restriction are energetic stressors that represent major challenges to organismal homeostasis, triggering wide-ranging responses in numerous cells and tissues controlling glucose and energy metabolism. An essential component of an organism’s survival is the ability to sense energy availability and to adapt accordingly. Metabolic flexibility, the ability to shift between fat and glucose oxidation with fasting and feeding, is reduced in individuals with metabolic diseases and contributes to the overall insulin resistance phenotype (Kelley et al., 1992). Skeletal muscle exhibits metabolic flexibility in fuel preference, likely due to its crucial role in hunting and surviving predation, situations requiring movement even if nutrient availability is not optimal (Freese et al., 2017). A body of literature supports the idea that metabolic flexibility can be directly influenced by physical activity, independent of changes in energy balance (Rynders et al., 2018). Physical exercise enhances skeletal muscle insulin sensitivity and improves whole-body glucose metabolism in people with type 2 diabetes (Savikj and Zierath, 2020). However, recent findings, based on stable-isotope tracer and liquid chromatography tandem mass spectrometry, demonstrate that skeletal muscle mitochondrial substrate preference is not altered in insulin resistant rodents and humans, calling into question the central role of metabolic flexibility in the pathogenesis of metabolic diseases (Song et al., 2020). Nevertheless, there is growing appreciation that insulin resistance, obesity, and type 2 diabetes can be avoided or at least delayed by lifestyle intervention strategies, including diet and exercise, which initiate diverse homeostatic responses across multiple organs (Savikj and Zierath, 2020).
The concept of “time-restricted feeding” has gained traction as a dietary means to restore metabolic homeostasis, enhance insulin sensitivity, and curb obesity. Time-restricted feeding refers to restricting daily food intake to a few hours, without caloric restriction (Chaix et al., 2014). In rodents, time-restricted feeding synchronizes the feeding/fasting cycle with the central clock, thereby promoting robust circadian and metabolic cycles, which mitigates obesity and metabolic dysfunction (Hatori et al., 2012). Thus, timing of food intake with the molecular circadian clock may fine-tune metabolism. In humans, time-restricted feeding paradigms improve cardiometabolic health in people with obesity or metabolic disease (Cienfuegos et al., 2020; Wilkinson et al., 2020). Short-term time-restricted feeding schedules in men with obesity modulate the diurnal rhythm of lipid and amino acid metabolism, without affecting core clock gene expression in skeletal muscle (Lundell et al., 2020). Furthermore, the timing and type of nutritional intake throughout a day influences carbohydrate metabolism and protein synthesis (Areta et al., 2013). Whether this is dependent upon the release of hormones, metabolites, or thermogenesis warrants further investigation. Moreover, the weight and cardiometabolic benefits achieved with time-restricted feeding schedules may be related to reductions in calorie intake, rather than meal timing. Concordantly, a prospective randomized clinical trial including 116 men and women with overweight or obesity found that modest reductions in weight loss and energy intake from time-restricted eating did not differ from the control group (Lowe et al., 2020), hinting at the possibility that benefits of time-restricted feeding programs are mainly due to reductions in calorie intake.
Diet and exercise have a synergistic effect on insulin sensitivity, which may be influenced by altering the timing of the meal or an exercise bout throughout the day. In rodents, there is a time-of-day-dependent effect of acute exercise on the diurnal oscillations of skeletal muscle metabolites and transcripts, with a greater reliance on glycolytic metabolism when exercise is performed during the early active phase of the day (Sato et al., 2019). Moreover, in a preliminary clinical investigation comparing the time-of-day impact of high intensity exercise in men with type 2 diabetes, greater blood glucose control was achieved with afternoon versus morning exercise (Savikj et al., 2019).
The oxygen-sensitive transcription factor HIF1α links time-of-day-specific effects of exercise on gene expression and carbohydrate metabolism in mice models (Peek et al., 2017; Sato et al., 2019). This finding has clinical relevance, since intense exercise acutely increases skeletal muscle protein abundance and DNA binding activity of HIF1α in humans (Ameln et al., 2005). Moreover, energetic stressors, such as exercise and hypoxia, increase skeletal muscle glucose uptake in healthy and insulin resistant humans and rodents (Ranheim et al., 1997; Ryder et al., 2000). Thus, perturbing energy, nutrient, and/or oxygen sensors may have a varied response on cellular metabolism depending on the time of day. Collectively, these studies provide evidence that the timing of exercise bouts throughout the day is clinically relevant for the diurnal control of glycemia or systemic metabolism. Adjusting the timing of external cues (i.e., meal/exercise timing) may sustain or amplify circadian clock signals to prevent or mitigate metabolic disease.
Thermal tolerance
Excess energy can be dissipated in the form of heat, a process that occurs in brown adipose tissue and is stimulated by food intake and cold exposure (Chouchani et al., 2019). Feedback loops involving temperature sensors, thermogenesis, sweating, and the control of blood circulation are tightly regulated to maintain body temperature in humans at ∼37°C. Alterations in ambient temperature trigger acute and chronic changes in whole-body physiology, making climate a major environmental stressor that affects all individuals on the planet. Acute exposure to cold triggers shivering in skeletal muscle, where ATP is used to generate movement and its associated production of heat. Chronic adaptation to cold involves different mechanisms, the main one being activation of brown adipose tissue thermogenesis (Chouchani et al., 2019). Uncoupling protein 1 dissipates the proton gradient in the mitochondria to generate heat instead of ATP. Consequently, oxidative phosphorylation increases to maintain mitochondrial membrane potential. Therefore, exposure to cold temperatures increases the metabolic rate during sleep cycles, as well as diet-induced thermogenesis, thereby increasing total energy expenditure (Chouchani et al., 2019). A rise in ambient temperature above thermoneutrality also increases metabolism by promoting heat dissipation (Chouchani et al., 2019).
The processes involved in heat acclimation have been extensively studied in humans and involve an increase in total body water, increased sweat volume and decreased sweat concentration, as well as adaptations of heart rate and skin blood flow (Périard et al., 2015). Mechanisms involved in heat acclimation and associated cardiovascular events are related to increased central heat production and dehydration and the ensuing deleterious consequences on blood pressure and cardiovascular function (Meade et al., 2020).
Acute exposure to extreme ambient temperatures, often referred to as “cold stress” or “heat stress,” is associated with an increased risk of cardio-pulmonary mortality (Achebak et al., 2019). In this context, age, weight, obesity, and type 2 diabetes are major risk factors (Hajat et al., 2017; Huang et al., 2012). The mechanisms for increased risk of cardiovascular events secondary to extreme temperatures in people with metabolic diseases are poorly understood. Reduced heat tolerance in obesity might be due to impairments in blood flow and sweat production (Vroman et al., 1983). The reduced sweating ability is possibly linked to a decreased body surface area-to-body mass ratio in a person with obesity as compared to a leaner person. Individuals with type 2 diabetes also exhibit reduced skin blood flow in response to local and whole-body heating, likely due to impaired endothelial function (Meade et al., 2020). However, chronic exposure to mild electrical stimulation with heat shock improves visceral adiposity, glucose homeostasis, and insulin sensitivity in people with type 2 diabetes (Kondo et al., 2014). This paradox suggests that increasing heat tolerance by repeated acute exposures to heat might mitigate heat-induced cardiovascular events in individuals with metabolic diseases.
Heat stress from both exercise and environmental factors can increase thermal strain in unacclimated individuals (Figure 5). Acute exercise increases core body temperature and high-intensity exercise can lead to heat illness consisting of symptoms ranging from minor cramps and syncope to major heat stroke, even in highly trained athletes (Charlot et al., 2017). The capacity to dissipate an exercise-induced elevation in body temperature is reduced in people with type 2 diabetes, but this can be overcome by regular exercise training, which is associated with improved heat tolerance (Kenny et al., 2016). Regular exercise training also reduces cardiovascular mortality and improves glucose control in people with type 2 diabetes (Savikj and Zierath, 2020). At a molecular level, exercise training increases heat shock protein abundance, a process that could contribute to the beneficial effects of exercise to enhance insulin sensitivity (Archer et al., 2018). Individuals with obesity or type 2 diabetes exhibit decreased levels of heat shock proteins in skeletal muscle (Chung et al., 2008). This decrease is reversible, and induction of heat shock proteins by mild electrical stimulation with heat shock improves visceral adiposity as well as plasma glucose and insulin levels (Kondo et al., 2014). Regular exposure to thermal stressors, such as exercise or environmental temperature, may improve heat tolerance through overlapping adaptive mechanistic responses (sweat volume and composition, body water, heart rate), thereby improving metabolism and decreasing risk of cardio-pulmonary events in individuals exposed to extreme ambient temperatures.
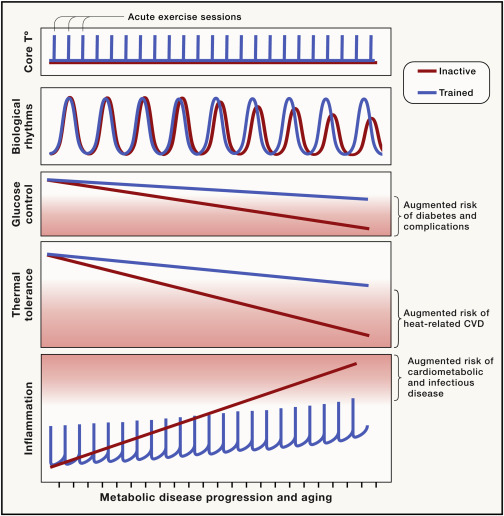
Figure 5. Beneficial effects of exercise training on metabolic risk
Decreased thermal tolerance, increased chronic inflammation, deregulated circadian rhythms, and poor glucose control worsen with age and disease development, increasing the susceptibility to life-threatening infections and extreme temperatures and eventually the risk of cardiovascular events (CVD). Exercise triggers acute and transient changes in inflammation and body temperature. These acute events are required for the beneficial effects of exercise training. Exercise training slows the progression of chronic inflammation, limits the decrease in heat-tolerance, and helps synchronize circadian clocks, thereby improving metabolism.
Presently, most of the human population lives under conditions of thermoneutrality, which is made possible due to appropriate clothing and heating systems in homes and workplaces. Reduced energy expenditure, due to the comforts of our modern society and the decline in our prolonged exposure to cold environments, may contribute to the worldwide rise in obesity, although this is difficult to firmly establish. However, there is a clear link between thermal regulation, metabolic diseases, and associated complications. Most of the temperature-related cardio-pulmonary events occur on moderately hot and moderately cold days (Gasparrini et al., 2015), suggesting that steady increases in the average global temperature has the potential to impact the numbers of these events worldwide. The combination of an epidemic of obesity and type 2 diabetes, juxtaposed with an aging population and climate change, may potentially lead to a dramatic increase in cardio-vascular morbimortality. Understanding the molecular basis of heat intolerance in people with obesity or type 2 diabetes could open novel preventative and therapeutic perspectives.
Inflammatory responses
The stress that temperature, obesity, diabetes, exercise, and food exert on organismal homeostasis triggers activation of the immune system and different states of metabolic inflammation. The immune system is composed of specialized cells present in every organ that protect against a wide variety of insults, including infections, mechanical injuries, and a variety of diseases. The immune response comes in waves, starting with a pro-inflammatory activation and finishing with a resolving anti-inflammatory phase (Feehan and Gilroy, 2019). When the immune system fails to recover after an insult, a chronic inflammatory state occurs, leading to long-term deleterious consequences. This typically happens in obesity, where immune cells infiltrate tissues and lead to chronic low-grade inflammation, associated with increased risk of cardiovascular complications. The association of inflammation with type 2 diabetes and obesity has been extensively studied, as evidenced by the rapid development of the field of “immunometabolism,” which includes the analysis of the complex interactions between metabolic and inflammatory pathways in immune and metabolic tissues (Lee et al., 2018).
Obesity and type 2 diabetes are associated with an accumulation of immune cells in key tissues involved in metabolic homeostasis. A link between metabolic diseases and immunology emerged with the detection of macrophage infiltration in adipose tissue, followed by the discovery that lymphocytes, neutrophils, and other specific subtypes of immune cells accumulate not only in adipose tissue but also in skeletal muscle and liver (Hotamisligil, 2017). Even neuroinflammation is part of the systemic inflammatory syndrome in metabolic diseases (Cai, 2013). The accumulation of triglycerides in adipocytes increases adipocyte size (hypertrophy) and number (hyperplasia), resulting in the rapid expansion of adipose tissue, which triggers hypoxia and the production of soluble mediators likely responsible for the attraction of immune cells. The first immune cells reaching adipose tissue are likely attracted to support tissue remodeling in a beneficial manner, but the chronic increase in adipose tissue volume and the establishment of a new obese steady-state leads to increasing lipolysis and circulating free fatty acids, which activate immune cells toward a pro-inflammatory phenotype and promote the establishment of chronic inflammation (Lee et al., 2018). Immune cells also respond to metabolic changes and are susceptible to the deleterious effects of an excessive lipid or glucose accumulation (i.e., “lipotoxicity” or “glucotoxicity”), as well as other metabolism-related danger signals that are released by tissues during metabolic stress (Wang et al., 2020b). The composition and phenotype of circulating immune cells is altered in blood of individuals with obesity, with an increase in CD16+ monocytes, and immune cell activation in response to high concentrations of glucose or fatty acids (Pillon et al., 2016). These findings suggest that the immune system is profoundly affected by whole-body glucose and lipid homeostasis.
The mechanisms by which non-adipose tissues establish a state of inflammation is unclear. However, lipotoxicity, including the excessive accumulation of toxic lipid mediators such as ceramides, diacylglycerol, or acylcarnitine, and increased levels of circulating free fatty acids likely play a role. In addition, activated immune cells primed to respond to metabolism-related danger signals can impair whole-body metabolism. There is ample evidence to suggest that inflammation is associated with the development of metabolic diseases and the ensuing complications, but pharmacological targeting of pathways controlling immunometabolism has shown limited benefits for the treatment of metabolic diseases (Pålsson-McDermott and O’Neill, 2020). Perhaps the key to successful clinical intervention will be to identify relevant patient groups early, before the manifestation of a chronic low-grade inflammatory state.
Acute exercise, especially intense and/or eccentric exercise, triggers an acute inflammatory response, which is necessary for skeletal muscle repair and adaptations to exercise training. Exercise training has beneficial anti-inflammatory effects (Gleeson et al., 2011). Thus, repeated peaks of inflammation triggered by acute bouts of moderate intensity exercise may be beneficial to reduce long-term basal concentrations of pro-inflammatory mediators. In severely obese individuals, combining exercise and dietary interventions can reduce macrophage infiltration and pro-inflammatory polarization in adipose tissue (Bruun et al., 2006). The anti-inflammatory effects of exercise could be secondary to an increased capacity for fatty acid utilization, as exercise training in people with obesity or type 2 diabetes reduces the level of deleterious lipid species such as DAG, acetylcarnitines, and ceramides in skeletal muscle (Lancaster and Febbraio, 2014). However, exercise training in healthy individuals also improves insulin sensitivity without changes in these lipid species, making the role of intramyocellular lipids on insulin sensitivity ambiguous and perhaps more relevant in an obesity context (Reidy et al., 2020).
Unsuspected causes
Currently known genetic, lifestyle, and environmental risk factors only partly explain the development of obesity and diabetes. Other yet unknown factors must be at play. A recent example of potential novel causes of diabetes is the high prevalence of extreme hyperglycemia/ketoacidosis in patients not known to have diabetes admitted to hospital with COVID-19 (Rubino et al., 2020). This seems both more common and more severe than has been seen with other infections/serious illnesses, so it may not represent “stress hyperglycemia” or unmasking of pre-existing, undiagnosed diabetes. Instead, these observations may suggest a specific pathological entity. The SARS-CoV-2 spike protein penetrates cell membranes by binding to the angiotensin converting enzyme (ACE) 2 receptor. This receptor is present on pancreatic beta cells (Hamming et al., 2004). Infection may result in acute loss of insulin secretory capacity and/or beta cell destruction (Apicella et al., 2020). ACE2 receptor is also present on adipocytes so that SARS-CoV-2 may also exacerbate chronic inflammation in adipose tissue (Kassir, 2020).
The mechanisms whereby widely accepted risk factors such as obesity result in disease may have novel aspects. It is generally assumed that individuals with type 2 diabetes who are not obese have a different pathophysiological cause unrelated to weight. However, this belief has been challenged recently. The concept of a “personal fat threshold” arose from observations that the median BMI in the UK Prospective Diabetes Study was only 28 kg/m2 (Taylor and Holman, 2015) and that reversal of type 2 diabetes by weight loss could be achieved equally successfully in individuals with higher and lower BMI (Lim et al., 2011). The underlying mechanism appears to be lipotoxicity, an individual’s propensity to accumulate liver and pancreas fat, and their susceptibility to the adverse effects of fat accumulation. At any given body weight or BMI, at-risk individuals will accumulate more liver fat and be more susceptible to developing hepatic insulin resistance at any given liver fat content. The subsequent increase in VLDL-TG export from the liver drives fat accumulation in the pancreas and declining insulin secretion, both also dependent on the individual’s susceptibility. Remission of type 2 diabetes by weight loss is accompanied by reduction in liver and pancreatic fat, decreased hepatic VLDL export, and increased insulin secretion (Al-Mrabeh et al., 2020). Conversely, weight re-gain leading to re-emergence of diabetes is associated with increased liver fat export and pancreatic fat, with recurrent pancreatic dysfunction. The importance of these observations underscores the usefulness of weight loss in the management of diabetic individuals even of normal weight. However, diabetes does not remit in everyone following substantial weight loss, so weight loss is not a universal panacea. Further work is needed to establish if this relates to longer duration diabetes, perhaps with irreversible beta cell damage, or to different pathological mechanisms of disease.
Bending the curve
Weight loss is clearly the key to reducing rates of obesity and type 2 diabetes, with considerable individual and societal benefits. There is a continuum of action required in prevention of obesity and diabetes, and management and care if they develop (Chan et al., 2020). Many intervention programs have demonstrated successful short-term weight loss and reversal of diabetes, but perhaps the bigger challenge is in preventing weight re-gain (Forouhi et al., 2018). There may be a weight “set-point,” at which compensatory hormonal, metabolic and neurochemical mechanisms prevent further weight loss and drive weight regain (Blüher, 2019). However, a significant proportion of individuals who lose a substantial amount of weight, whether by diet or bariatric surgery, do not regain weight over years and maintain the metabolic benefits of the initial weight loss. Thus, weight loss programs must have two parts: an initial phase of weight loss, followed by a weight maintenance program. Obviously, reduction in energy intake by some means is essential for weight loss. Exactly how this is achieved is probably less important than an individual’s ability to adhere to the program long term (Johnston et al., 2014). The benefits of one regimen over another have been debated (Forouhi et al., 2018), but no one size fits all, and many different approaches are needed.
Understanding the influence of social and cultural aspects in the development and management of obesity and diabetes is also crucial (Blüher, 2019). Individuals from socially deprived backgrounds are more likely to be at high risk to develop obesity and type 2 diabetes, to have poorer glycemic control, to develop more complications, and to have a greater reduction in life expectancy (Chan et al., 2020). Identifying and overcoming barriers to participation in screening and prevention programs and in diabetes and obesity care are vital. Most programs do not reach individuals from ethnic minorities or low socioeconomic class, who are most at need (Timpel et al., 2019). Involving overweight and obese individuals from a wide diversity of backgrounds in the identification of barriers to adherence and then in the design of weight loss and diabetes prevention/reversal programs is essential to improve engagement.
Personalized medicine
Although we talk about type 2 diabetes as one disease, this “blanket” diagnosis covers important heterogeneity (Ahlqvist et al., 2018). Only rarely is the heterogeneity obvious and explainable: slim rather than obese, or young age at presentation with a striking family history in monogenic diabetes. On many occasions, individuals with apparently similar phenotypes have very different clinical courses and respond quite differently to glucose lowering agents. Dissecting out particular forms of “type 2 diabetes,” whether by genetic analyses and risk scores or by improving our understanding of the underlying pathophysiological bases to dysglycemia, is currently possible at the population level but remains extremely difficult at an individual level. As a result, the selection of glucose-lowering agents for individuals is a “best guess” approach, far removed from personalized medicine.
Personalized medicine is defined simply as the right treatment for the right person at the right time. The recent American Diabetes Association/European Association for the Study of Diabetes consensus report describes the ambition to personalize all aspects of an individual’s diabetes, including precision diagnosis, lifestyle and pharmacological management, and prognosis (Chung et al., 2020). Currently, for a very small number of individuals (for example, those with congenital leptin deficiency [Montague et al., 1997] and GCK-MODY [MODY 2] [Froguel et al., 1992; Hattersley et al., 1992]), precision diagnosis is possible. However, there are major challenges in precision diagnosis for individuals with the polygenic common forms of obesity and type 2 diabetes. Likewise, there are only a small number of examples of precision therapeutics (for example, leptin for management of severe obesity in congenital leptin deficiency [Farooqi et al., 2002] and sulphonylureas rather than insulin for individuals with neonatal diabetes due to mutations in the genes encoding the potassium channel [KCNJ11 and ABCC8] [Pearson et al., 2006]). For many individuals with obesity and type 2 diabetes, we have extremely blunt “precision” tools. For example, analysis of data from participants in the RECORD and ADOPT trials demonstrated that individuals with insulin resistance have a greater sustained fall in HbA1c on thiazolidenediones compared to sulphonylureas (Dennis et al., 2018). Additionally, there are benefits of SGLT2 inhibitors in individuals with high cardiovascular risk and/or renal disease (Lo et al., 2020). Work is beginning to examine possibilities for personalization of lifestyle measures.
Grand challenges
Prevention of obesity is probably the most important factor in reducing the prevalence of obesity and related metabolic diseases. This will require action at an individual and societal level (Chan et al., 2020). Societal action is necessary in many areas, including changes to road, rail, and cycling transport plans to encourage increased physical activity, as well as negotiations with the food industry (Chan et al., 2020). Governments also need effective communication plans that reach all sections of society (Timpel et al., 2019). Moreover, different strategies are required for different life stages. The lifestyles of almost everyone must change radically, and this must be facilitated by appropriate action by governments and many branches of industry. The challenges to overcome the status quo and vested interests are considerable.
There is abundant evidence that many individuals with obesity at high risk of metabolic disease can lose substantial amounts of weight, reversing pre-diabetes and diabetes. A significant proportion then maintain the weight loss and improved metabolic status for years. Weight loss programs are projected to be more effective per quality-adjusted life year and cost-saving over a lifetime compared to standard care in individuals with type 2 diabetes (Xin et al., 2020). The challenge then is to expand and adapt these successful programs so that all individuals can access them and be supported through them. We must work with individuals who find current programs inappropriate for them, identifying barriers to participation and working together to develop practical solutions. Most current weight loss programs center on improving basic diet, with or without advice on exercise (Forouhi et al., 2018). Further incremental benefit may well be obtained by incorporating additional “personalized” measures, perhaps based on genes, occupation, and inflammatory status, such as advice on specific micronutrients, timing of food intake and exercise, and light exposure. However, the challenge will be to ensure that the message does not become so complex that adherence falls.
There is a particular challenge for young people (Chan et al., 2020). The WHO estimated in 2016 that world-wide, 340 million children and adolescents aged 5–19 years were overweight or obese and, in 2019, that 38 million children aged <5 years were overweight/obese (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Associated with this, type 2 diabetes is increasingly diagnosed in children, adolescents, and young adults (IDF Diabetes Atlas 9th edition 2019, www.diabetesatlas.org). A recent meta-analysis has demonstrated the greater impact of type 2 diabetes presenting at younger age: each one-year increase in age at diabetes diagnosis was associated with a 4%, 3%, and 5% decreased risk of all-cause mortality, macrovascular, and microvascular disease respectively (Nanayakkara et al., 2020). These changes underscore the need to prevent obesity and/or manage it appropriately in young people.
Paralleling the rise in obesity in younger people is the rise in the number of women with hyperglycemia during pregnancy. The IDF estimated that, in 2017, 16% of women with live births had some form of hyperglycemia during pregnancy, and that 86% of them had gestational diabetes (IDF Diabetes Atlas 9th edition 2019, www.diabetesatlas.org). In addition to the immediate maternal and fetal adverse effects of hyperglycemia during pregnancy, many of these women will develop type 2 diabetes in the subsequent 5-10 years. There are also longer-term consequences to the offspring of increased risk of obesity, type 2 diabetes, hypertension, and cardiovascular disease (Catalano and Shankar, 2017). Some of these adverse consequences are now being reported over several generations of offspring, implicating an epigenetic influence (Catalano and Shankar, 2017). Thus, in addition to the immediate management of the index pregnancy, it is extremely important that further studies of the index in women, their children, and potentially subsequent generations are conducted urgently.
Low levels of fitness are a risk factor for hospitalizations and all-cause mortality, and predict morbidity after surgical interventions (West et al., 2016). During the COVID-19 pandemic, public health recommendations regarding confinement and closure of recreation areas decreased daily activity in the general population (Sánchez-Sánchez et al., 2020), aggravating already high levels of inactivity in most countries (https://www.who.int/news-room/fact-sheets/detail/physical-activity). In young adults who only developed mild symptoms, COVID-19 decreased the predicted maximal aerobic capacity (Crameri et al., 2020), while persons more severely infected with SARS-CoV-2 exhibited anorexia and skeletal muscle loss, aggravated by long hospital stays, raising the question of whether COVID-19 could be a major cause of cachexia and sarcopenia (Morley et al., 2020). As obesity and type 2 diabetes are risk factors for COVID-19 complications, the underlying inflammatory conditions in combination with impaired skeletal muscle function may contribute to worse outcomes after infection (Guisado-Vasco et al., 2020). Whether exercise can protect against viral infection or influence disease severity is unclear, but the benefits of physical activity to prevent skeletal muscle wasting are important factors for prevention and rehabilitation of people in risks groups. Understanding the molecular mechanisms underlying the beneficial effects of physical exercise as an inflammatory modulator could thus potentially prevent or mitigate complications due to unexpected infections (da Silveira et al., 2021; Krause et al., 2020).
On the horizon
How do we move forward? Progress will only come if we tackle the problems at both a population and an individual level. Putting into practice what we already know will benefit many individuals (Chan et al., 2020). Incorporating the newer evidence described above—for example, around the timing of eating and exercise—and light exposure, in ways that do not overwhelm people, will bring added benefits. Better personalization of all aspects of prevention, management, and care should help adherence. In-depth large-scale analysis of genetic and environmental factors may help clarify why people respond differently to the whole gamut of care, allow stratification into refined sub-groups with specific risk factors and genetic predispositions, and potentially thus optimize the efficacy of both lifestyle and pharmacological interventions. Ongoing initiatives like the Innovative Medicines Initiative (www.imi.europa.eu) have demonstrated that combining large databases from multiple public and private organizations is possible, generating power to tackle relevant genetic and biomarker questions. Such initiatives, bringing together diverse stakeholders with people with obesity or diabetes, are essential in our efforts to provide personalized, timely, affordable, and equitable access to high-quality health interventions, with the aim of improving health outcomes for all.
Acknowledgments
N.J.P. was supported by an Individual Fellowship from the Marie Skłodowska-Curie Actions (European Commission, 704978). J.R.Z. was supported from the Swedish Research Council (Vetenskapsrådet) (2015-00165), a Novo Nordisk Foundation Challenge Grant (NNF14OC0011493), and the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (NNF18CC0034900). R.J.F.L. was supported by the National Institutes of Health (R01DK110113; R01DK107786; R01HL142302; R01 DK124097) and the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (Alliance 190503).
Author contributions
N.J.P., R.L., S.M., and J.R.Z. wrote the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests. J.R.Z. is member of Cell Advisory Board.
References
- Achebak et al., 2019
- H. Achebak, D. Devolder, J. Ballester
Trends in temperature-related age-specific and sex-specific mortality from cardiovascular diseases in Spain: a national time-series analysis
Lancet Planet. Health, 3 (2019), pp. e297-e306
- Ahlqvist et al., 2018
- E. Ahlqvist, P. Storm, A. Käräjämäki, M. Martinell, M. Dorkhan, A. Carlsson, P. Vikman, R.B. Prasad, D.M. Aly, P. Almgren, et al.
Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables
Lancet Diabetes Endocrinol., 6 (2018), pp. 361-369
- Al-Mrabeh et al., 2020
- A. Al-Mrabeh, S.V. Zhyzhneuskaya, C. Peters, A.C. Barnes, S. Melhem, A. Jesuthasan, B. Aribisala, K.G. Hollingsworth, G. Lietz, J.C. Mathers, et al.
Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss
Cell Metab., 31 (2020), pp. 233-249 e234
- Ameln et al., 2005
- H. Ameln, T. Gustafsson, C.J. Sundberg, K. Okamoto, E. Jansson, L. Poellinger, Y. Makino
Physiological activation of hypoxia inducible factor-1 in human skeletal muscle
FASEB J., 19 (2005), pp. 1009-1011
- American Diabetes Association, 2020
- American Diabetes Association
2. Classification and diagnosis of diabetes: Standards of medical care in Diabetes-2020
Diabetes Care, 43 (Suppl 1) (2020), pp. S14-S31
- Apicella et al., 2020
- M. Apicella, M.C. Campopiano, M. Mantuano, L. Mazoni, A. Coppelli, S. Del Prato
COVID-19 in people with diabetes: understanding the reasons for worse outcomes
Lancet Diabetes Endocrinol., 8 (2020), pp. 782-792
- Archer et al., 2018
- A.E. Archer, A.T. Von Schulze, P.C. Geiger
Exercise, heat shock proteins and insulin resistance
Philos. Trans. R. Soc. Lond. B Biol. Sci., 373 (2018), 10.1098/rstb.2016.0529
- Areta et al., 2013
- J.L. Areta, L.M. Burke, M.L. Ross, D.M. Camera, D.W. West, E.M. Broad, N.A. Jeacocke, D.R. Moore, T. Stellingwerff, S.M. Phillips, et al.
Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis
J. Physiol., 591 (2013), pp. 2319-2331
- Balsalobre et al., 2000
- A. Balsalobre, S.A. Brown, L. Marcacci, F. Tronche, C. Kellendonk, H.M. Reichardt, G. Schütz, U. Schibler
Resetting of circadian time in peripheral tissues by glucocorticoid signaling
Science, 289 (2000), pp. 2344-2347
- Barrès and Zierath, 2016
- R. Barrès, J.R. Zierath
The role of diet and exercise in the transgenerational epigenetic landscape of T2DM
Nat. Rev. Endocrinol., 12 (2016), pp. 441-451
- Barrès et al., 2012
- R. Barrès, J. Yan, B. Egan, J.T. Treebak, M. Rasmussen, T. Fritz, K. Caidahl, A. Krook, D.J. O’Gorman, J.R. Zierath
Acute exercise remodels promoter methylation in human skeletal muscle
Cell Metab., 15 (2012), pp. 405-411
- Barres et al., 2013
- R. Barres, H. Kirchner, M. Rasmussen, J. Yan, F.R. Kantor, A. Krook, E. Näslund, J.R. Zierath
Weight loss after gastric bypass surgery in human obesity remodels promoter methylation
Cell Rep., 3 (2013), pp. 1020-1027
- Barron et al., 2020
- E. Barron, C. Bakhai, P. Kar, A. Weaver, D. Bradley, H. Ismail, P. Knighton, N. Holman, K. Khunti, N. Sattar, et al.
Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study
Lancet Diabetes Endocrinol., 8 (2020), pp. 813-822
- Barroso and McCarthy, 2019
- I. Barroso, M.I. McCarthy
The genetic basis of metabolic disease
Cell, 177 (2019), pp. 146-161
- Blüher, 2019
- M. Blüher
Obesity: global epidemiology and pathogenesis
Nat. Rev. Endocrinol., 15 (2019), pp. 288-298
- Bošković and Rando, 2018
- A. Bošković, O.J. Rando
Transgenerational epigenetic inheritance
Annu. Rev. Genet., 52 (2018), pp. 21-41
- Bray et al., 2017
- G.A. Bray, K.K. Kim, J.P.H. Wilding, World Obesity Federation
Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation
Obes. Rev., 18 (2017), pp. 715-723
- Bruun et al., 2006
- J.M. Bruun, J.W. Helge, B. Richelsen, B. Stallknecht
Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects
Am. J. Physiol. Endocrinol. Metab., 290 (2006), pp. E961-E967
- Cai, 2013
- D. Cai
Neuroinflammation and neurodegeneration in overnutrition-induced diseases
Trends Endocrinol. Metab., 24 (2013), pp. 40-47
- Catalano and Shankar, 2017
- P.M. Catalano, K. Shankar
Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child
BMJ, 356 (2017), p. j1
- Chaix et al., 2014
- A. Chaix, A. Zarrinpar, P. Miu, S. Panda
Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges
Cell Metab., 20 (2014), pp. 991-1005
- Chan et al., 2020
- J.C.N. Chan, L.L. Lim, N.J. Wareham, J.E. Shaw, T.J. Orchard, P. Zhang, E.S.H. Lau, B. Eliasson, A.P.S. Kong, M. Ezzati, et al.
The Lancet Commission on diabetes: using data to transform diabetes care and patient lives
Lancet, 396 (10267) (2020), pp. 2019-2082, 10.1016/S0140-6736(20)32374-6
- Charlot et al., 2017
- K. Charlot, C. Faure, S. Antoine-Jonville
Influence of hot and cold environments on the regulation of energy balance following a single exercise session: A mini-review
Nutrients, 9 (2017), p. 9
- Chouchani et al., 2019
- E.T. Chouchani, L. Kazak, B.M. Spiegelman
New advances in adaptive thermogenesis: UCP1 and beyond
Cell Metab., 29 (2019), pp. 27-37
- Chung et al., 2008
- J. Chung, A.K. Nguyen, D.C. Henstridge, A.G. Holmes, M.H. Chan, J.L. Mesa, G.I. Lancaster, R.J. Southgate, C.R. Bruce, S.J. Duffy, et al.
HSP72 protects against obesity-induced insulin resistance
Proc. Natl. Acad. Sci. USA, 105 (2008), pp. 1739-1744
- Chung et al., 2020
- W.K. Chung, K. Erion, J.C. Florez, A.T. Hattersley, M.F. Hivert, C.G. Lee, M.I. McCarthy, J.J. Nolan, J.M. Norris, E.R. Pearson, et al.
Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)
Diabetologia, 63 (2020), pp. 1671-1693
- Cienfuegos et al., 2020
- S. Cienfuegos, K. Gabel, F. Kalam, M. Ezpeleta, E. Wiseman, V. Pavlou, S. Lin, M.L. Oliveira, K.A. Varady
Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: A Randomized Controlled Trial in Adults with Obesity.
Cell Metab., 32 (2020), pp. 366-378.e363
- Crameri et al., 2020
- G.A.G. Crameri, M. Bielecki, R. Züst, T.W. Buehrer, Z. Stanga, J.W. Deuel
Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020
Euro Surveill., 25 (2020), p. 2001542
- Crosby et al., 2019
- P. Crosby, R. Hamnett, M. Putker, N.P. Hoyle, M. Reed, C.J. Karam, E.S. Maywood, A. Stangherlin, J.E. Chesham, E.A. Hayter, et al.
Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time
Cell, 177 (2019), pp. 896-909 e820
- da Silveira et al., 2021
- M.P. da Silveira, K.K. da Silva Fagundes, M.R. Bizuti, E. Starck, R.C. Rossi, E.S.D.T. de Resende
Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature
Clin. Exp. Med., 21 (2021), pp. 15-28, 10.1007/s10238-020-00650-3
- de Castro Barbosa et al., 2015
- T. de Castro Barbosa, L.R. Ingerslev, P.S. Alm, S. Versteyhe, J. Massart, M. Rasmussen, I. Donkin, R. Sjögren, J.M. Mudry, L. Vetterli, et al.
High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring
Mol. Metab., 5 (2015), pp. 184-197
- Dennis et al., 2018
- J.M. Dennis, B.M. Shields, A.G. Jones, E.R. Pearson, A.T. Hattersley, W.E. Henley, MASTERMIND consortium
Evaluating associations between the benefits and risks of drug therapy in type 2 diabetes: a joint modeling approach
Clin. Epidemiol., 10 (2018), pp. 1869-1877
- Elks et al., 2012
- C.E. Elks, M. den Hoed, J.H. Zhao, S.J. Sharp, N.J. Wareham, R.J. Loos, K.K. Ong
Variability in the heritability of body mass index: a systematic review and meta-regression
Front. Endocrinol. (Lausanne), 3 (2012), p. 29
- Farooqi et al., 2002
- I.S. Farooqi, G. Matarese, G.M. Lord, J.M. Keogh, E. Lawrence, C. Agwu, V. Sanna, S.A. Jebb, F. Perna, S. Fontana, et al.
Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency
J. Clin. Invest., 110 (2002), pp. 1093-1103
- Feehan and Gilroy, 2019
- K.T. Feehan, D.W. Gilroy
Is resolution the end of inflammation?
Trends Mol. Med., 25 (2019), pp. 198-214
- Forouhi et al., 2018
- N.G. Forouhi, A. Misra, V. Mohan, R. Taylor, W. Yancy
Dietary and nutritional approaches for prevention and management of type 2 diabetes
BMJ, 361 (2018), p. k2234
- Freese et al., 2017
- J. Freese, R.J. Klement, B. Ruiz-Núñez, S. Schwarz, H. Lötzerich
The sedentary (r)evolution: Have we lost our metabolic flexibility?
F1000Res., 6 (2017), p. 1787
- Froguel et al., 1992
- P. Froguel, M. Vaxillaire, F. Sun, G. Velho, H. Zouali, M.O. Butel, S. Lesage, N. Vionnet, K. Clément, F. Fougerousse, et al.
Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus
Nature, 356 (1992), pp. 162-164
- Gabriel and Zierath, 2019
- B.M. Gabriel, J.R. Zierath
Circadian rhythms and exercise - re-setting the clock in metabolic disease
Nat. Rev. Endocrinol., 15 (2019), pp. 197-206
- Gamble et al., 2014
- K.L. Gamble, R. Berry, S.J. Frank, M.E. Young
Circadian clock control of endocrine factors
Nat. Rev. Endocrinol., 10 (2014), pp. 466-475
- Gasparrini et al., 2015
- A. Gasparrini, Y. Guo, M. Hashizume, E. Lavigne, A. Zanobetti, J. Schwartz, A. Tobias, S. Tong, J. Rocklöv, B. Forsberg, et al.
Mortality risk attributable to high and low ambient temperature: a multicountry observational study
Lancet, 386 (2015), pp. 369-375
- Gleeson et al., 2011
- M. Gleeson, N.C. Bishop, D.J. Stensel, M.R. Lindley, S.S. Mastana, M.A. Nimmo
The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease
Nat. Rev. Immunol., 11 (2011), pp. 607-615
- Guisado-Vasco et al., 2020
- P. Guisado-Vasco, M. Cano-Megías, M. Rodríguez-López, I.M. de-Luna-Boquera, D. Carnevali-Ruiz, Immunosuppressants Against COVID-19 Working Team
COVID-19 and metabolic syndrome: NF-κB activation. Crossroads
Trends Endocrinol. Metab., 31 (2020), pp. 802-803
- Hajat et al., 2017
- S. Hajat, A. Haines, C. Sarran, A. Sharma, C. Bates, L.E. Fleming
The effect of ambient temperature on type-2-diabetes: case-crossover analysis of 4+ million GP consultations across England
Environ. Health, 16 (2017), p. 73
- Hamming et al., 2004
- I. Hamming, W. Timens, M.L. Bulthuis, A.T. Lely, G. Navis, H. van Goor
Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis
J. Pathol., 203 (2004), pp. 631-637
- Hatori et al., 2012
- M. Hatori, C. Vollmers, A. Zarrinpar, L. DiTacchio, E.A. Bushong, S. Gill, M. Leblanc, A. Chaix, M. Joens, J.A. Fitzpatrick, et al.
Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet
Cell Metab., 15 (2012), pp. 848-860
- Hattersley and Patel, 2017
- A.T. Hattersley, K.A. Patel
Precision diabetes: learning from monogenic diabetes
Diabetologia, 60 (2017), pp. 769-777
- Hattersley et al., 1992
- A.T. Hattersley, R.C. Turner, M.A. Permutt, P. Patel, Y. Tanizawa, K.C. Chiu, S. O’Rahilly, P.J. Watkins, J.S. Wainscoat
Linkage of type 2 diabetes to the glucokinase gene
Lancet, 339 (1992), pp. 1307-1310
- Holman et al., 2020
- N. Holman, P. Knighton, P. Kar, J. O’Keefe, M. Curley, A. Weaver, E. Barron, C. Bakhai, K. Khunti, N.J. Wareham, et al.
Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study
Lancet Diabetes Endocrinol., 8 (2020), pp. 823-833
- Hotamisligil, 2017
- G.S. Hotamisligil
Inflammation, metaflammation and immunometabolic disorders
Nature, 542 (2017), pp. 177-185
- Huang et al., 2012
- C. Huang, A.G. Barnett, X. Wang, S. Tong
Effects of extreme temperatures on years of life lost for cardiovascular deaths: a time series study in Brisbane, Australia
Circ. Cardiovasc. Qual. Outcomes, 5 (2012), pp. 609-614
- Ji et al., 2019
- Y. Ji, A.M. Yiorkas, F. Frau, D. Mook-Kanamori, H. Staiger, E.L. Thomas, N. Atabaki-Pasdar, A. Campbell, J. Tyrrell, S.E. Jones, et al.
Genome-wide and abdominal MRI data provide evidence that a genetically determined favorable adiposity phenotype is characterized by lower ectopic liver fat and lower risk of type 2 diabetes, heart disease, and hypertension
Diabetes, 68 (2019), pp. 207-219
- Johnston et al., 2014
- B.C. Johnston, S. Kanters, K. Bandayrel, P. Wu, F. Naji, R.A. Siemieniuk, G.D. Ball, J.W. Busse, K. Thorlund, G. Guyatt, et al.
Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis
JAMA, 312 (2014), pp. 923-933
- Kaati et al., 2002
- G. Kaati, L.O. Bygren, S. Edvinsson
Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period
Eur. J. Hum. Genet., 10 (2002), pp. 682-688
- Kassir, 2020
- R. Kassir
Risk of COVID-19 for patients with obesity
Obes. Rev., 21 (2020), p. e13034
- Kelley et al., 1992
- D.E. Kelley, M. Mokan, L.J. Mandarino
Intracellular defects in glucose metabolism in obese patients with NIDDM
Diabetes, 41 (1992), pp. 698-706
- Kenny et al., 2016
- G.P. Kenny, R.J. Sigal, R. McGinn
Body temperature regulation in diabetes
Temperature (Austin), 3 (2016), pp. 119-145
- Kettner et al., 2015
- N.M. Kettner, S.A. Mayo, J. Hua, C. Lee, D.D. Moore, L. Fu
Circadian dysfunction induces leptin resistance in mice
Cell Metab., 22 (2015), pp. 448-459
- Khera et al., 2019
- A.V. Khera, M. Chaffin, K.H. Wade, S. Zahid, J. Brancale, R. Xia, M. Distefano, O. Senol-Cosar, M.E. Haas, A. Bick, et al.
Polygenic prediction of weight and obesity trajectories from birth to adulthood
Cell, 177 (2019), pp. 587-596.e589
- Kim and Lazar, 2020
- Y.H. Kim, M.A. Lazar
Transcriptional control of circadian rhythms and metabolism: A matter of time and space
Endocr. Rev., 41 (2020), pp. 707-732
- Kohner et al., 1998
- E.M. Kohner, S.J. Aldington, I.M. Stratton, S.E. Manley, R.R. Holman, D.R. Matthews, R.C. Turner
United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors
Arch. Ophthalmol., 116 (1998), pp. 297-303
- Kondo et al., 2014
- T. Kondo, K. Ono, S. Kitano, R. Matsuyama, R. Goto, M.A. Suico, S. Kawasaki, M. Igata, J. Kawashima, H. Motoshima, et al.
Mild electrical stimulation with heat shock reduces visceral adiposity and improves metabolic abnormalities in subjects with metabolic syndrome or type 2 diabetes: Randomized Crossover Trials
EBioMedicine, 1 (2014), pp. 80-89
- Koopman et al., 2017
- A.D.M. Koopman, S.P. Rauh, E. van ’t Riet, L. Groeneveld, A.A. van der Heijden, P.J. Elders, J.M. Dekker, G. Nijpels, J.W. Beulens, F. Rutters
The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study
J. Biol. Rhythms, 32 (2017), pp. 359-368
- Krause et al., 2020
- M. Krause, F. Gerchman, R. Friedman
Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: is heat shock response determinant for the disease complications?
Diabetol. Metab. Syndr., 12 (2020), p. 63
- Lamia et al., 2009
- K.A. Lamia, U.M. Sachdeva, L. DiTacchio, E.C. Williams, J.G. Alvarez, D.F. Egan, D.S. Vasquez, H. Juguilon, S. Panda, R.J. Shaw, et al.
AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation
Science, 326 (2009), pp. 437-440
- Lancaster and Febbraio, 2014
- G.I. Lancaster, M.A. Febbraio
The immunomodulating role of exercise in metabolic disease
Trends Immunol., 35 (2014), pp. 262-269
- Larder et al., 2017
- R. Larder, M.F.M. Sim, P. Gulati, R. Antrobus, Y.C.L. Tung, D. Rimmington, E. Ayuso, J. Polex-Wolf, B.Y.H. Lam, C. Dias, et al.
Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation
Proc. Natl. Acad. Sci. USA, 114 (2017), pp. 9421-9426
- Lee et al., 2018
- Y.S. Lee, J. Wollam, J.M. Olefsky
An integrated view of immunometabolism
Cell, 172 (2018), pp. 22-40
- Li et al., 2010
- Y. Li, Y. He, L. Qi, V.W. Jaddoe, E.J. Feskens, X. Yang, G. Ma, F.B. Hu
Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood
Diabetes, 59 (2010), pp. 2400-2406
- Lim et al., 2011
- E.L. Lim, K.G. Hollingsworth, B.S. Aribisala, M.J. Chen, J.C. Mathers, R. Taylor
Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol
Diabetologia, 54 (2011), pp. 2506-2514
- Lin et al., 2009
- Y.C. Lin, T.J. Hsiao, P.C. Chen
Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up
Chronobiol. Int., 26 (2009), pp. 740-755
- Lo et al., 2020
- K.B. Lo, F. Gul, P. Ram, A.Y. Kluger, K.M. Tecson, P.A. McCullough, J. Rangaswami
The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: A Systematic Review and Meta-Analysis
Cardiorenal Med., 10 (2020), pp. 1-10
- Locke et al., 2015
- A.E. Locke, B. Kahali, S.I. Berndt, A.E. Justice, T.H. Pers, F.R. Day, C. Powell, S. Vedantam, M.L. Buchkovich, J. Yang, et al., LifeLines Cohort Study, ADIPOGen Consortium, AGEN-BMI Working Group, CARDIOGRAMplusC4D Consortium, CKDGen Consortium, GLGC, ICBP, MAGIC Investigators, MuTHER Consortium, MIGen Consortium, PAGE Consortium, ReproGen Consortium, GENIE Consortium, International Endogene Consortium
Genetic studies of body mass index yield new insights for obesity biology
Nature, 518 (2015), pp. 197-206
- Lowe et al., 2020
- D.A. Lowe, N. Wu, L. Rohdin-Bibby, A.H. Moore, N. Kelly, Y.E. Liu, E. Philip, E. Vittinghoff, S.B. Heymsfield, J.E. Olgin, et al.
Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: The TREAT Randomized Clinical Trial
JAMA Intern. Med., 180 (2020), pp. 1491-1499
- Lundell et al., 2020
- L.S. Lundell, E.B. Parr, B.L. Devlin, L.R. Ingerslev, A. Altıntaş, S. Sato, P. Sassone-Corsi, R. Barrès, J.R. Zierath, J.A. Hawley
Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression
Nat. Commun., 11 (2020), p. 4643
- Mahajan et al., 2018
- A. Mahajan, D. Taliun, M. Thurner, N.R. Robertson, J.M. Torres, N.W. Rayner, A.J. Payne, V. Steinthorsdottir, R.A. Scott, N. Grarup, et al.
Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps
Nat. Genet., 50 (2018), pp. 1505-1513
- Meade et al., 2020
- R.D. Meade, A.P. Akerman, S.R. Notley, R. McGinn, P. Poirier, P. Gosselin, G.P. Kenny
Physiological factors characterizing heat-vulnerable older adults: A narrative review
Environ. Int., 144 (2020), p. 105909
- Montague et al., 1997
- C.T. Montague, I.S. Farooqi, J.P. Whitehead, M.A. Soos, H. Rau, N.J. Wareham, C.P. Sewter, J.E. Digby, S.N. Mohammed, J.A. Hurst, et al.
Congenital leptin deficiency is associated with severe early-onset obesity in humans
Nature, 387 (1997), pp. 903-908
- Morley et al., 2020
- J.E. Morley, K. Kalantar-Zadeh, S.D. Anker
COVID-19: a major cause of cachexia and sarcopenia?
J. Cachexia Sarcopenia Muscle, 11 (2020), pp. 863-865
- Multhaup et al., 2015
- M.L. Multhaup, M.M. Seldin, A.E. Jaffe, X. Lei, H. Kirchner, P. Mondal, Y. Li, V. Rodriguez, A. Drong, M. Hussain, et al.
Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes
Cell Metab., 21 (2015), pp. 138-149
- Murashov et al., 2016
- A.K. Murashov, E.S. Pak, M. Koury, A. Ajmera, M. Jeyakumar, M. Parker, O. Williams, J. Ding, D. Walters, P.D. Neufer
Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice
FASEB J., 30 (2016), pp. 775-784
- Nanayakkara et al., 2020
- N. Nanayakkara, A.J. Curtis, S. Heritier, A. Gadowski, M.E. Pavkov, T. Kenealy, D.R. Owens, R.L. Thomas, S. Song, J. Wong, et al.
Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses
Diabetologia, 64 (2020), pp. 275-287, 10.1007/s00125-020-05319-w
Published online December 14, 2020
- Newman and Maddocks, 2017
- A.C. Newman, O.D.K. Maddocks
One-carbon metabolism in cancer
Br. J. Cancer, 116 (2017), pp. 1499-1504
- Nitert et al., 2012
- M.D. Nitert, T. Dayeh, P. Volkov, T. Elgzyri, E. Hall, E. Nilsson, B.T. Yang, S. Lang, H. Parikh, Y. Wessman, et al.
Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes
Diabetes, 61 (2012), pp. 3322-3332
- Pålsson-McDermott and O’Neill, 2020
- E.M. Pålsson-McDermott, L.A.J. O’Neill
Targeting immunometabolism as an anti-inflammatory strategy
Cell Res., 30 (2020), pp. 300-314
- Pan et al., 2011
- A. Pan, E.S. Schernhammer, Q. Sun, F.B. Hu
Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women
PLoS Med., 8 (2011), p. e1001141
- Pearson et al., 2006
- E.R. Pearson, I. Flechtner, P.R. Njølstad, M.T. Malecki, S.E. Flanagan, B. Larkin, F.M. Ashcroft, I. Klimes, E. Codner, V. Iotova, et al., Neonatal Diabetes International Collaborative Group
Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations
N. Engl. J. Med., 355 (2006), pp. 467-477
- Peek et al., 2017
- C.B. Peek, D.C. Levine, J. Cedernaes, A. Taguchi, Y. Kobayashi, S.J. Tsai, N.A. Bonar, M.R. McNulty, K.M. Ramsey, J. Bass
Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle
Cell Metab., 25 (2017), pp. 86-92
- Périard et al., 2015
- J.D. Périard, S. Racinais, M.N. Sawka
Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports
Scand. J. Med. Sci. Sports, 25 (Suppl 1) (2015), pp. 20-38
- Pillon et al., 2016
- N.J. Pillon, K.L. Chan, S. Zhang, M. Mejdani, M.R. Jacobson, A. Ducos, P.J. Bilan, W. Niu, A. Klip
Saturated fatty acids activate caspase-4/5 in human monocytes, triggering IL-1β and IL-18 release
Am. J. Physiol. Endocrinol. Metab., 311 (2016), pp. E825-E835
- Ranheim et al., 1997
- T. Ranheim, C. Dumke, K.L. Schueler, G.D. Cartee, A.D. Attie
Interaction between BTBR and C57BL/6J genomes produces an insulin resistance syndrome in (BTBR x C57BL/6J) F1 mice
Arterioscler. Thromb. Vasc. Biol., 17 (1997), pp. 3286-3293
- Rathjen et al., 2017
- T. Rathjen, X. Yan, N.L. Kononenko, M.C. Ku, K. Song, L. Ferrarese, V. Tarallo, D. Puchkov, G. Kochlamazashvili, S. Brachs, et al.
Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1
Nat. Neurosci., 20 (2017), pp. 1096-1103
- Ravelli et al., 1998
- A.C. Ravelli, J.H. van der Meulen, R.P. Michels, C. Osmond, D.J. Barker, C.N. Hales, O.P. Bleker
Glucose tolerance in adults after prenatal exposure to famine
Lancet, 351 (1998), pp. 173-177
- Reidy et al., 2020
- P.T. Reidy, Z.S. Mahmassani, A.I. McKenzie, J.J. Petrocelli, S.A. Summers, M.J. Drummond
Influence of exercise training on skeletal muscle insulin resistance in aging: Spotlight on muscle ceramides
Int. J. Mol. Sci., 21 (2020), p. 1514
- GBD 2019 Risk Factors Collaborators, 2020
- GBD 2019 Risk Factors Collaborators
Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019
Lancet, 396 (2020), pp. 1223-1249
- Rubino et al., 2020
- F. Rubino, S.A. Amiel, P. Zimmet, G. Alberti, S. Bornstein, R.H. Eckel, G. Mingrone, B. Boehm, M.E. Cooper, Z. Chai, et al.
New-onset diabetes in Covid-19
N. Engl. J. Med., 383 (2020), pp. 789-790
- Rudic et al., 2004
- R.D. Rudic, P. McNamara, A.M. Curtis, R.C. Boston, S. Panda, J.B. Hogenesch, G.A. Fitzgerald
BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis
PLoS Biol., 2 (2004), p. e377
- Ryder et al., 2000
- J.W. Ryder, J. Yang, D. Galuska, J. Rincón, M. Björnholm, A. Krook, S. Lund, O. Pedersen, H. Wallberg-Henriksson, J.R. Zierath, G.D. Holman
Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients
Diabetes, 49 (2000), pp. 647-654
- Rynders et al., 2018
- C.A. Rynders, S. Blanc, N. DeJong, D.H. Bessesen, A. Bergouignan
Sedentary behaviour is a key determinant of metabolic inflexibility
J. Physiol., 596 (2018), pp. 1319-1330
- Sánchez-Sánchez et al., 2020
- E. Sánchez-Sánchez, G. Ramírez-Vargas, Y. Avellaneda-López, J.I. Orellana-Pecino, E. García-Marín, J. Díaz-Jimenez
Eating habits and physical activity of the Spanish population during the COVID-19 pandemic period
Nutrients, 12 (2020), p. 2826
- Sato et al., 2019
- S. Sato, A.L. Basse, M. Schonke, S. Chen, M. Samad, A. Altintas, R.C. Laker, E. Dalbram, R. Barres, P. Baldi, et al.
Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis.
Cell Metab., 30 (2019), pp. 92-110.e114
- Savikj and Zierath, 2020
- M. Savikj, J.R. Zierath
Train like an athlete: applying exercise interventions to manage type 2 diabetes
Diabetologia, 63 (2020), pp. 1491-1499
- Savikj et al., 2019
- M. Savikj, B.M. Gabriel, P.S. Alm, J. Smith, K. Caidahl, M. Björnholm, T. Fritz, A. Krook, J.R. Zierath, H. Wallberg-Henriksson
Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial
Diabetologia, 62 (2019), pp. 233-237
- Song et al., 2020
- J.D. Song, T.C. Alves, D.E. Befroy, R.J. Perry, G.F. Mason, X.M. Zhang, A. Munk, Y. Zhang, D. Zhang, G.W. Cline, et al.
Dissociation of muscle insulin resistance from alterations in mitochondrial substrate preference
Cell Metab., 32 (2020), pp. 726-735.e725
- Stanford et al., 2015
- K.I. Stanford, M.Y. Lee, K.M. Getchell, K. So, M.F. Hirshman, L.J. Goodyear
Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring
Diabetes, 64 (2015), pp. 427-433
- Taylor and Holman, 2015
- R. Taylor, R.R. Holman
Normal weight individuals who develop type 2 diabetes: the personal fat threshold
Clin. Sci. (Lond.), 128 (2015), pp. 405-410
- Tiffon, 2018
- C. Tiffon
The Impact of nutrition and environmental epigenetics on human health and disease
Int. J. Mol. Sci., 19 (2018), p. 3425
- Timpel et al., 2019
- P. Timpel, L. Harst, D. Reifegerste, S. Weihrauch-Blüher, P.E.H. Schwarz
What should governments be doing to prevent diabetes throughout the life course?
Diabetologia, 62 (2019), pp. 1842-1853
- Turek et al., 2005
- F.W. Turek, C. Joshu, A. Kohsaka, E. Lin, G. Ivanova, E. McDearmon, A. Laposky, S. Losee-Olson, A. Easton, D.R. Jensen, et al.
Obesity and metabolic syndrome in circadian Clock mutant mice
Science, 308 (2005), pp. 1043-1045
- Udler et al., 2018
- M.S. Udler, J. Kim, M. von Grotthuss, S. Bonàs-Guarch, J.B. Cole, J. Chiou, M. Boehnke, M. Laakso, G. Atzmon, B. Glaser, et al., Christopher D. Anderson on behalf of METASTROKE and the ISGC
Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis
PLoS Med., 15 (2018), p. e1002654
- Udler et al., 2019
- M.S. Udler, M.I. McCarthy, J.C. Florez, A. Mahajan
Genetic risk scores for diabetes diagnosis and precision medicine
Endocr. Rev., 40 (2019), pp. 1500-1520
- van der Klaauw and Farooqi, 2015
- A.A. van der Klaauw, I.S. Farooqi
The hunger genes: pathways to obesity
Cell, 161 (2015), pp. 119-132
- Vimalananda et al., 2015
- V.G. Vimalananda, J.R. Palmer, H. Gerlovin, L.A. Wise, J.L. Rosenzweig, L. Rosenberg, E.A. Ruiz Narváez
Night-shift work and incident diabetes among African-American women
Diabetologia, 58 (2015), pp. 699-706
- Vroman et al., 1983
- N.B. Vroman, E.R. Buskirk, J.L. Hodgson
Cardiac output and skin blood flow in lean and obese individuals during exercise in the heat
J. Appl. Physiol., 55 (1983), pp. 69-74
- Wang et al., 2020a
- S. Wang, P. Ma, S. Zhang, S. Song, Z. Wang, Y. Ma, J. Xu, F. Wu, L. Duan, Z. Yin, et al.
Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study
Diabetologia, 63 (2020), pp. 2102-2111
- Wang et al., 2020b
- X. Wang, Y. Wang, V. Antony, H. Sun, G. Liang
Metabolism-associated molecular patterns (MAMPs)
Trends Endocrinol. Metab., 31 (2020), pp. 712-724
ArticleDownload PDFCrossRefView Record in ScopusGoogle Scholar
- West et al., 2016
- M.A. West, R. Asher, M. Browning, G. Minto, M. Swart, K. Richardson, L. McGarrity, S. Jack, M.P. Grocott, Perioperative Exercise Testing and Training Society
Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery
Br. J. Surg., 103 (2016), pp. 744-752
- Wilkinson et al., 2020
- M.J. Wilkinson, E.N.C. Manoogian, A. Zadourian, H. Lo, S. Fakhouri, A. Shoghi, X. Wang, J.G. Fleischer, S. Navlakha, S. Panda, et al.
Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome
Cell Metab., 31 (2020), pp. 92-104.e105
- Willemsen et al., 2015
- G. Willemsen, K.J. Ward, C.G. Bell, K. Christensen, J. Bowden, C. Dalgård, J.R. Harris, J. Kaprio, R. Lyle, P.K. Magnusson, et al.
The concordance and heritability of type 2 diabetes in 34,166 twin pairs from international twin registers: The Discordant Twin (Dros. Inf. Serv.COTWIN) Consortium
Twin Res. Hum. Genet., 18 (2015), pp. 762-771
- Xin et al., 2020
- Y. Xin, A. Davies, A. Briggs, L. McCombie, C.M. Messow, E. Grieve, W.S. Leslie, R. Taylor, M.E.J. Lean
Type 2 diabetes remission: 2 year within-trial and lifetime-horizon cost-effectiveness of the Diabetes Remission Clinical Trial (DiRECT)/Counterweight-Plus weight management programme
Diabetologia, 63 (2020), pp. 2112-2122
- Yengo et al., 2018
- L. Yengo, J. Sidorenko, K.E. Kemper, Z. Zheng, A.R. Wood, M.N. Weedon, T.M. Frayling, J. Hirschhorn, J. Yang, P.M. Visscher, GIANT Consortium
Meta-analysis of genome-wide association studies for height and body mass index in :700000 individuals of European ancestry
Hum. Mol. Genet., 27 (2018), pp. 3641-3649
- Young, 2018
- M.W. Young
Time travels: A 40-Year journey from drosophila’s clock mutants to human circadian disorders (Nobel Lecture)
Angew. Chem. Int. Ed. Engl., 57 (2018), pp. 11532-11539
- Yuan and Larsson, 2020
- S. Yuan, S.C. Larsson
An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study
Diabetologia, 63 (2020), pp. 2359-2371



