5.3: Energy - Photophosphorylation
- Page ID
- 8614
Source: BiochemFFA_5_3.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy

Photophosphorylation
The third type of phosphorylation to make ATP is found only in cells that carry out photosynthesis. This process is similar to oxidative phosphorylation in several ways. A primary difference is the ultimate source of the energy for ATP synthesis. In oxidative phosphorylation, the energy comes from electrons produced by oxidation of biological molecules. In photosynthesis, the energy comes from the light of the sun. Photons from the sun interact with chlorophyll molecules in reaction centers in the chloroplasts (Figures \(\PageIndex{1}\) and \(\PageIndex{2}\)) of plants or membranes of photosynthetic bacteria.
The similarities of photophosphorylation to oxidative phosphorylation include:
- a membrane associated electron transport chain
- creation of a proton gradient
- harvesting energy of the proton gradient by making ATP with the help of an ATP synthase.
Some of the differences include :
- the source of the electrons – H2O for photosynthesis versus NADH/FADH2 for oxidative phosphorylation
- direction of proton pumping – into the thylakoid space of the chloroplasts versus outside the matrix of the mitochondrion
- movement of protons during ATP synthesis – out of the thylakoid space in photosynthesis versus into the mitochondrial matrix in oxidative phosphorylation
- nature of the terminal electron acceptor – NADP+ in photosynthesis versus O2 in oxidative phosphorylation.
Electron transport: chloroplasts vs mitochondria
In some ways, the movement of electrons in chloroplasts during photosynthesis is opposite that of electron transport in mitochondria. In photosynthesis, water is the source of electrons and their final destination is NADP+ to make NADPH. In mitochondria, NADH/FADH2 are electron sources and H2O is their final destination. How do biological systems get electrons to go both ways? It would seem to be the equivalent of going to and from a particular place while always going downhill, since electrons will move according to potential.
Solar power
The answer is the captured energy of the photons from the sun (Figure 5.59), which elevates electrons to an energy where they move “downhill” to their NADPH destination in a Z-shaped scheme. The movement of electrons through this scheme in plants requires energy from photons in two places to “lift” the energy of the electrons sufficiently.
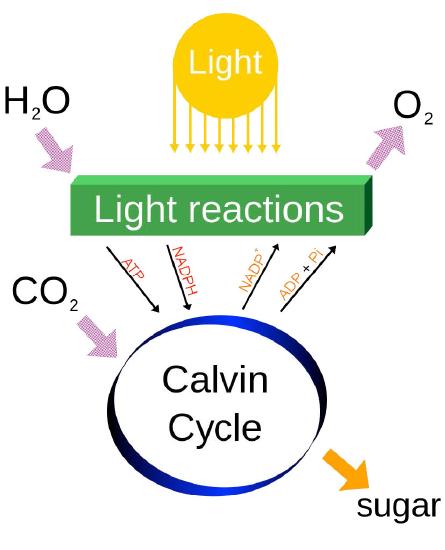
Last, it should be noted that photosynthesis actually has two phases, referred to as the light cycle (described above) and the dark cycle, which is a set of chemical reactions that captures CO2 from the atmosphere and “fixes” it, ultimately into glucose. The dark cycle is also referred to as the Calvin Cycle and is discussed HERE.
Photosynthesis
Photosynthesis is an energy capture process found in plants and other organisms to harvest light energy and convert it into chemical energy. This photochemical energy is stored ultimately in carbohydrates which are made using ATP (from the energy harvesting), carbon dioxide and water. In most cases, a byproduct of the process is oxygen, which is released from water in the capture process. Photosynthesis is responsible for most of the oxygen in the atmosphere and it supplies the organic materials and most of the energy used by life on Earth.
Steps
The steps in the photosynthesis process varies slightly between organisms. In a broad overview, it always starts with energy capture from light by protein complexes, containing chlorophyll pigments, called reaction centers. Plants sequester these proteins in chloroplasts, but bacteria, which don’t have organelles, embed them in their plasma membranes.
Energy from the light is used to strip electrons away from electron donors (usually water) and leave a byproduct (oxygen, if water was used). Electrons are donated to a carrier and ultimately are accepted by NADP+, to become NADPH. As electrons travel towards NADP+, they generate a proton gradient across the thylakoid membrane, which is used to drive synthesis of ATP. Thus NADPH, ATP, and oxygen are the products of the first phase of photosynthesis called the light reactions. Energy from ATP and electrons from NADPH are used to reduce CO2 and build sugars, which are the ultimate energy storage directly arising from photosynthesis.
Chloroplasts
Chloroplasts are found in almost all aboveground plant cells, but are primarily concentrated in leaves. The interior of a leaf, below the epidermis is made up of photosynthesis tissue called mesophyll, which can contain up to 800,000 chloroplasts per square millimeter.
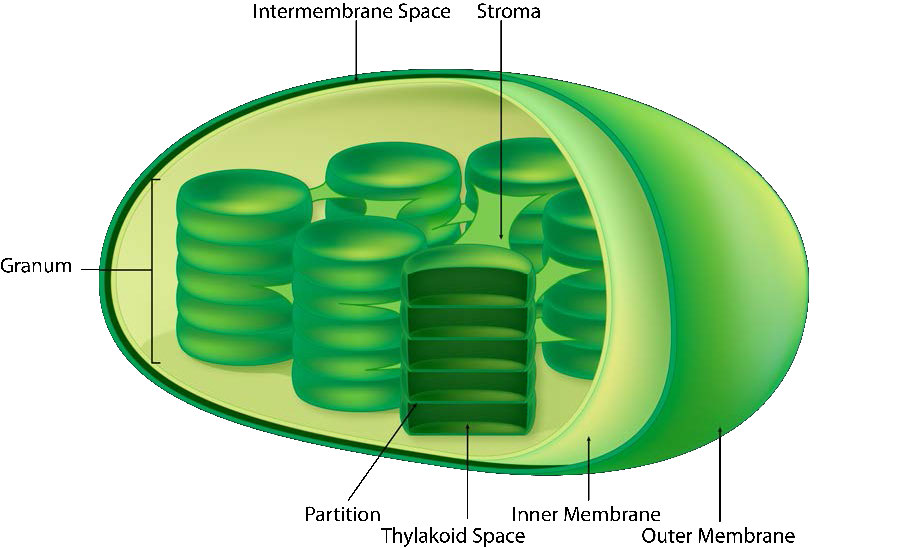
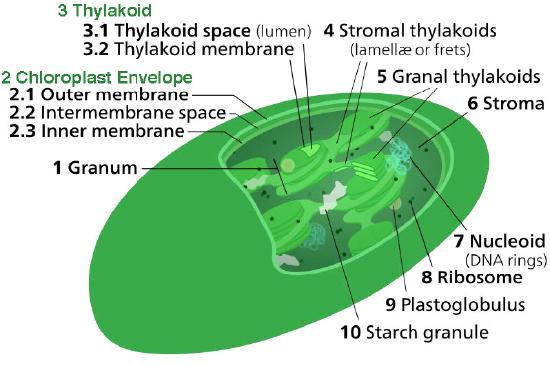
The chloroplast’s membrane has a phospholipid inner membrane, a phospholipid outer membrane, and a region between them called the intermembrane space (Figure 5.61). Within the inner chloroplast membrane is the stroma, in which the chloroplast DNA and the enzymes of the Calvin cycle are located. Also within the stroma are stacked, flattened disks known as thylakoids which are defined by their thylakoid membranes. The space within the thylakoid membranes are termed the thylakoid spaces or thylakoid lumen. The protein complexes containing the light-absorbing pigments, known as photosystems, are located on the thylakoid membrane. Besides chlorophylls, carotenes and xanthophylls are also present, allowing for absorption of light energy over a wider range. The same pigments are used by green algae and land plants.
Brown algae and diatoms add fucoxanthin (a xanthophyll) and red algae add phycoerythrin to the mix. In plants and algae, the pigments are held in a very organized fashion complexes called antenna proteins that help funnel energy, through resonance energy transfer, to the reaction center chlorophylls. A system so organized is called a light harvesting complex. The electron transport complexes of photosynthesis are also located on the thylakoid membranes.
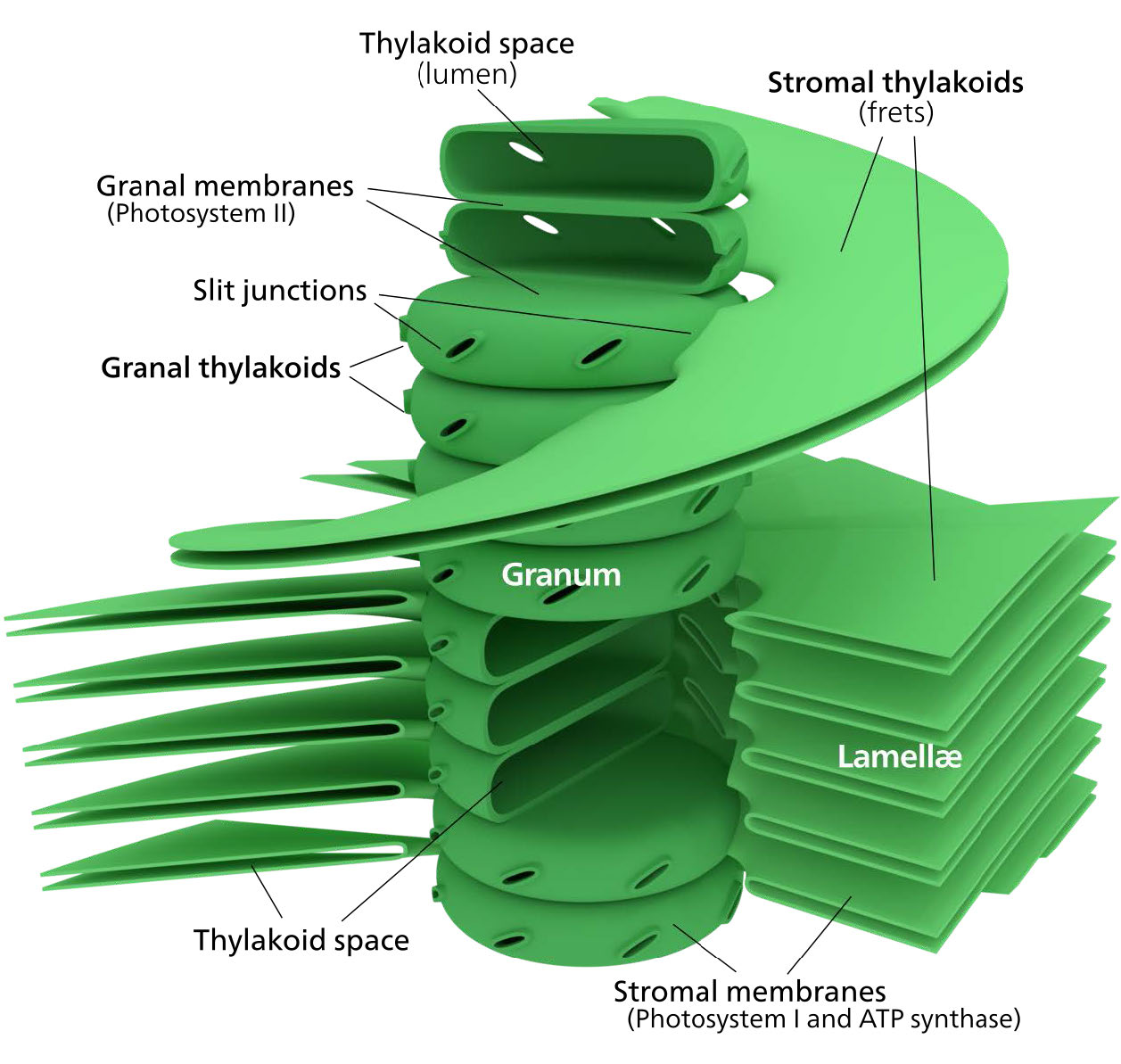
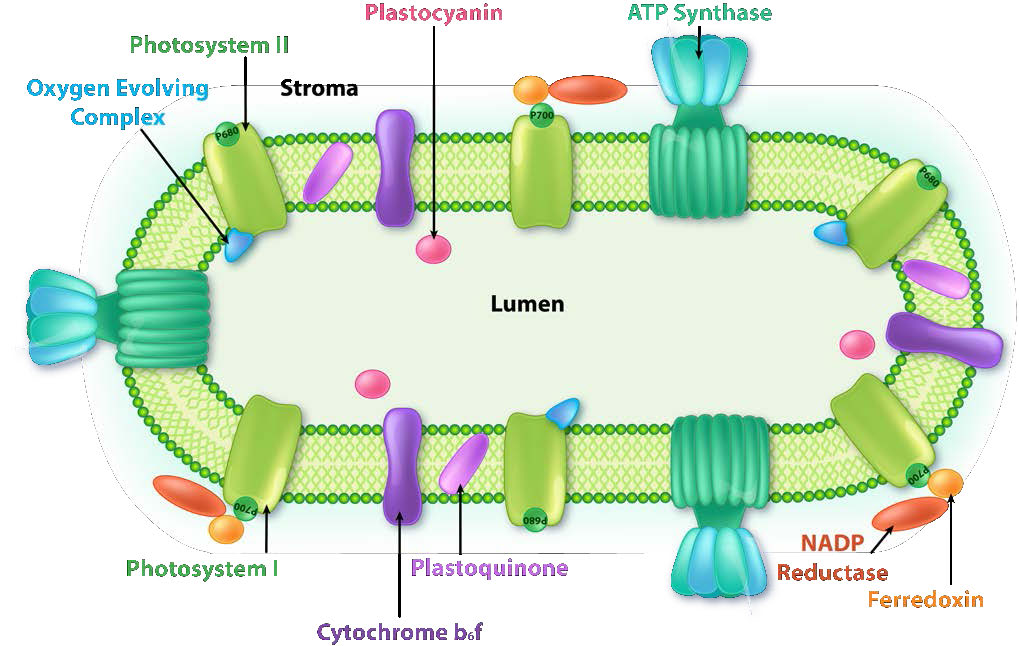
Figure \(\PageIndex{6}\): Complexes in the thylakoid membrane. Image by Aleia Kim
Light reactions of photosynthesis
In chloroplasts, the light reactions of photosynthesis involving electron transfer occur in the thylakoid membranes (Figure \(\PageIndex{6}\)). Separate biochemical reactions involving the assimilation of carbon dioxide to make glucose are referred to as the Calvin cycle, also sometimes referred to as the “dark reactions”. This will be discussed elsewhere in the section on metabolism (HERE).
The chloroplasts are where the energy of light is captured, electrons are stripped from water, oxygen is liberated, electron transport occurs, NADPH is formed, and ATP is generated. The thylakoid membrane corresponds to the inner membrane of the mitochondrion for transport of electrons and proton pumping (Figure \(\PageIndex{4}\)).
The thylakoid membrane does its magic using four major protein complexes. These include Photosystem II (PS II), Cytochrome b6f complex (Cb6f), Photosystem I (PS I), and ATP synthase. The roles of these complexes, respectively, are to capture light energy, create a proton gradient from electron movement, capture light energy (again), and use proton gradient energy from the overall process to synthesize ATP.
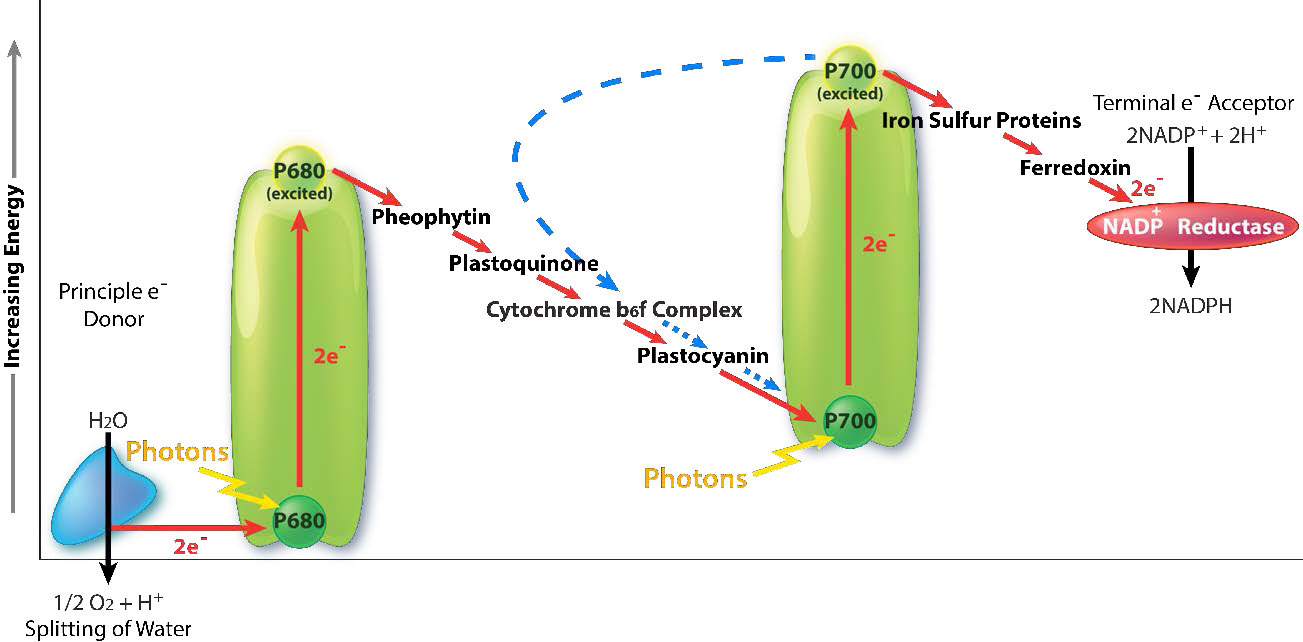
Light harvesting
Harvesting the energy of light begins in PS II with the absorption of a photon of light at a reaction center. PS II performs this duty best with light at a wavelength of 680 nm and it readily loses an electron to excitation when this occurs, leaving PS II with a positive charge. This electron must be replaced. The ultimate replacement source of electrons is water, but water must lose four electrons and PS II can only accept one at a time.
Manganese centers
An intermediate Oxygen Evolving Complex (OEC) contains four manganese centers that provide the immediate replacement electron that PSII requires. After four electrons have been donated by the OEC to PS II, the OEC extracts four electrons from two water molecules, liberating oxygen and dumping four protons into the thylakoid space, thus contributing to the proton gradient. The excited electron from PS II must be passed to another carrier very quickly, lest it decay back to its original state. It does this, giving its electron within picoseconds to pheophytin (Figure \(\PageIndex{8}\)).
Pheophytin passes the electron on to protein-bound plastoquinones . The first is known as PQA. PQA hands the electron off to a second plastoquinone (PQB), which waits for a second electron and collects two protons to become PQH2, also known as plastoquinol (Figure \(\PageIndex{9}\)). PQH2 passes these to the Cytochrome b6f complex (Cb6f) which uses passage of electrons through it to pump protons into the thylakoid space. ATP synthase makes ATP from the proton gradient created in this way. Cb6f drops the electron off at plastocyanin, which holds it until the next excitation process begins with absorption of another photon of light at 700 nm by PS I.
Absorption of light at PS I
With absorption of a photon of light by PS I, a process begins, that is similar to the process in PS II. PS I gains a positive charge as a result of the loss of an excited electron and pulls the electron in plastocyanin away from it. Meanwhile, the excited electron from PS I passes through an iron-sulfur protein, which gives the electron to ferredoxin (another iron sulfur protein). Ferredoxin then passes the electron off to the last protein in the system known as Ferredoxin:NADP+ oxidoreductase, which gives the electron and a proton to NADP+, creating NADPH.
Note that reduction of NADP+ to NADPH requires two electrons and one proton, so the four electrons and two protons from oxidation of water will result in production of two molecules of NADPH. At this point, the light cycle is complete - water has been oxidized, ATP has been created, and NADPH has been made. The electrons have made their way from water to NADPH via carriers in the thylakoid membrane and their movement has released sufficient energy to make ATP. Energy for the entire process came from four photons of light.
The two photosystems performing all of this magic are protein complexes that are similar in structure and means of operation. They absorb photons with high efficiency so that whenever a pigment in the photosynthetic reaction center absorbs a photon, an electron from the pigment is excited and transferred to another molecule almost instantaneously. This reaction is called photo-induced charge separation and it is a unique means of transforming light energy into chemical forms.
Cyclic photophosphorylation
Besides the path described above for movement of electrons through PS I, plants have an alternative route that electrons can take. Instead of electrons going through ferredoxin to form NADPH, they instead take a backwards path through the the proton-pumping b6f complex. This system, called cyclic photophosphorylation (Figure \(\PageIndex{8}\)) which generates more ATP and no NADPH, is similar to a system found in green sulfur bacteria. The ability of plants to switch between non-cyclic and cyclic photosystems allows them to make the proper ratio of ATP and NADPH they need for assimilation of carbon in the dark phase of photosynthesis. This ratio turns out to be 3 ATPs to 2 NADPHs.

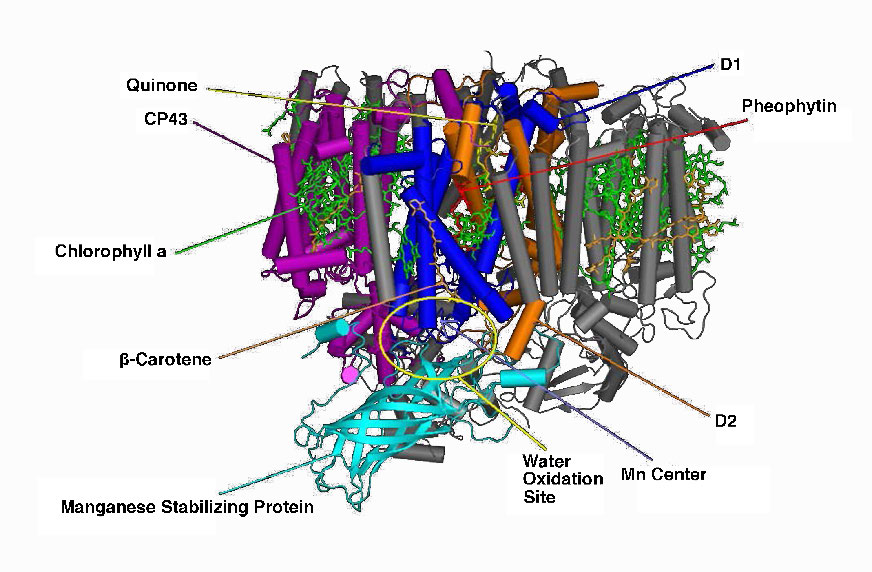
Figure \(\PageIndex{9}\) - Photosystem II of cyanobacteria. Wikipedia
Photosynthetic energy
The output of the photophosphorylation part of photosynthesis (O2, NADPH, and ATP), of course, is not the end of the process of photosynthesis. For the growing plant, the NADPH and ATP are used to capture carbon dioxide from the atmosphere and convert it (ultimately) into glucose and other important carbon compounds. This, as noted previously, occurs in the Calvin Cycle (see HERE) in what is called the dark phase of the process. The oxygen liberated in the process is a necessary for respiration of all aerobic life forms on Earth. Indeed, it is believed that essentially all of the oxygen in the atmosphere today is the result the splitting of water in photosynthesis over the many eons that the process has existed.


