Cells
- Page ID
- 2852
Time
The following parts of this lab should be done first because they will require time after they are set up. They should be done in the order shown below.
- Osmosis in Potato Cells takes 60 minutes after the experiment is set up. Click the link or scroll to go to that section of the lab.
- An Artificial Cell takes 40 minutes after it is set up.
- Diffusion in a Liquid takes the rest of the lab period after it is set up.
After you have begun the three parts listed above, you should complete the rest of the lab (below
Introduction
All organisms are composed of cells. Cells are the smallest structures that are living; they are the unit of life. Two very different kinds of cells exist in nature. Prokaryotes are the simplest kind of organisms (example: bacteria). Their cells lack many of the structures (organelles) typically found in more complex cells. All other organisms contain cells that are considerably more complex. These organisms include all of the plants, animals, fungi, and protists.
We will look at prokaryotic cells in this exercise but we will examine details of the structure of eukaryotic cells.
The plasma membrane is vitally important in regulating the passage of materials into and out of the cell. We will see that small cells have a large surface to volume ratio, thus, more plasma membrane to service it's contents.
The plasma membrane is differentially permeable, that is, some molecules such as water can pass through but others cannot. We will study some characteristics that result from this property.
Examination of Prokaryotic Cells
The exercises below require the use of a microscope. Click here for instructions on using the microscope.
- Examine a slide of bacterial types under high power (400 X). The slide contains spherical cells (cocci), rod-shaped cells (bacilli), and spiral shaped cells (spirilla). Draw several cells. The rod-shaped bacteria on the slide are attached end-to-end forming threadlike filaments. If you look carefully, you can seed the individual cells that compose the filament. Write the magnification used next to your diagram. Note the size of these cells compared to eukaryotic cells.
- Cyanobacteria are photosynthetic prokaryotes and may be connected in chains or filaments. Examine a slide of Cyanobacteria such as Anabaena under high power (400X). Draw representative Cyanobacteria in your notebook.
Examination of Eukaryotic Cells
We will examine an organism called amoeba as an example of a eukaryotic cell.
- Prepare a slide of live Amoeba. Use a dropper to obtain a sample from the bottom of the culture jar. There may be a wheat seed on the bottom of the jar. Try to obtain a drop from the bottom near the seed. If live Amoeba are not available, observe a prepared slide of Amoeba.
- Identify the pseudopodia. If you are also viewing a prepared slide that has been stained, you should also be able to see the nucleus. Note that the cell is much larger than the prokaryotic cells (above) and is filled with numerous organelles. The functions of some of these organelles will be discussed later.
- Draw an Amoeba in your notebook and indicate the magnification used.
Observation of a Living Plant Cell
- Prepare a wet mount of an Elodea leaf. View the cell under low and high power. Use the fine focus to focus up and down on a cell. Cells above and below your cell may interfere with your viewing. Identify the cell wall, and chloroplasts. If your specimen is fresh, you should be able to see the chloroplasts moving within the cell.
- Notice that there are few chloroplasts in the center of the cell. This space is occupied by the central vacuole.
- Draw an Elodea cell and state the magnification used.
Below: Elodea 100X and 400X.
 |
 |
Click on the photographs to view enlargements. |
Animal Cells
- If you have not observed human cheek cells in a previous laboratory exercise prepare a wet mount by using the following procedure.
Scrape the inside of your cheek with a toothpick and rub it on a dry slide.
Add one drop of methylene blue to stain the cells. This will make them easier to see.
Place a cover slip on the slide as described above and observe the cells under low power then high power.
- Identify the nucleus.
- How do these animal cells differ from the Elodea (plant) cells? See your drawings of typical plant and animal cells to help with the answer to this question.
- Draw a cheek cell.
Below: Human cheek cells 100X. Click on the photograph to view an enlargement.
Diffusion and Osmosis
Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration. The movement is due to molecular collisions, which occur more frequently in areas of higher concentration.
Diffusion
Diffusion in a Liquid
- Create a hypothesis for the experiment described below.
- Obtain a Petri dish and add enough water to cover the bottom.
- Place the dish on a ruler so that the metric scale crosses the center of the dish.
- Allow the water to remain still for one minute, then add a crystal of potassium permanganate to the center of the dish.
- Measure how far the molecules diffused after 10 minutes. Distance should be measured from the crystal to the edge of the purple area (or diameter of the purple area/2).
- Calculate the rate of diffusion per hour.
- Observe the dish at the end of the laboratory period. Did the potassium permanganate diffuse throughout the entire dish by the end of the laboratory period?
- After this part is set up, you should return to the top of this page and complete the rest of this lab.
Diffusion in a Gelatin
Several drops of dye have been added to tubes containing a clear gelatin.
- Obtain one of these tubes that was prepared during the past week and measure how far the dye diffused.
- Record the number of hours that the dye has been diffusing.
- Calculate the rate of diffusion per hour.
- Obtain a tube that had been prepared last semester. What happened?
| Dye was added to the top tube 3 days before the photograph was taken. Dye was added to the middle tube 157 days prior to photographing and the bottom tube was 370 days. How does the rate of diffusion in a gelatin compare to your experiment with diffusion in water? |
Osmosis
The center of cell membranes contains the nonpolar fatty acid tails of phospholipid molecules. Because of this large nonpolar area, charged particles and large polar molecules cannot diffuse across the membrane. Small polar molecules such as water can diffuse across the membrane.
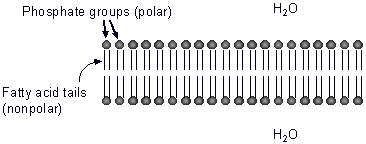
Osmosis is the diffusion of water across a differentially permeable membrane (see "Diffusion" above).
It occurs when a solute (example: salt, sugar, protein, etc.) cannot pass through a membrane but the solvent (water) can. Water always moves from where it is most concentrated (has less solute) to where it is less concentrated.
In general, water moves toward the area with a higher solute concentration because it has a lower water concentration.
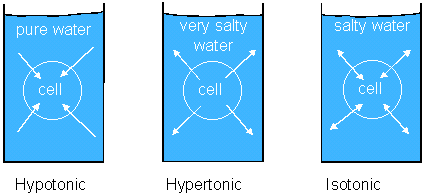
In the container on the left side of the diagram, water will enter the cell because it is more concentrated on the outside. In the center drawing, water is more concentrated inside the cell, so it will move out. If the solute concentration is the same inside as it is out, the amount of water that moves out will be approximately to the amount that moves in.
Osmotic pressure is the force of osmosis. In the diagram above, the cell on the left will swell. The pressure within the cell is osmotic pressure.
Artificial Cells
Dialysis tubing can be used to simulate the selective permeability of cell membranes and to demonstrate osmosis. Pores within the tubing restrict the passage of large molecules but allow water molecules to pass through. In biological membranes, large polar molecules such as cannot cross the polar region of the lipid bilayer but small molecules such as water can pass through. Sugar molecules are used in the experiment below because they are large and cannot pass through the tubing.
- Create a hypothesis for the experiment described below.
- Cut two pieces of dialysis tubing approximately 15 cm in length each.
- Moisten the tubes with water and then clamp one end. Plastic or foam clamps may be used. Instructions for using the foam clamps are below. Click an image to view an enlargement.
|
Twist the end of the dialysis tubing several times as shown in the photograph. |
 |
| Next, fold the twisted area as shown |  |
| Insert the folded end into the foam clamp. |  |
- Rinse the tubes under water so that they can be opened.
- Fill one tube 1/2 full with 50% molasses solution.
- Fill the other tube 1/2 full with deionized water.
- Clamp the other end of each tube. Be sure that you leave plenty of room in the tube for water to enter.
- Rinse each tube under water and let them drip for about 10 seconds to remove excess moisture.
- Place a plastic weighing tray on the scale and zero the scale. Weigh each tube to the nearest 0.1 g. Be sure to zero the scale and weighing tray before weighing the second tube. Do not place the bag directly on the metal weighing pan of the scale and do not drip liquids on the scale because this could damage the scale.
- Place the tube containing molasses in a beaker containing deionized water. The beaker should contain enough water to cover the tube.
- Place the tube containing deionized water in a beaker containing a concentrated sucrose (sugar) solution. Use enough sucrose solution to cover the tube.
- Weigh each tuber again after 10, 20, 30 and 40 minutes. Be sure to use a plastic weighing tray and to zero the scale before placing the tube on the weighing tray.
- Record your data in a table in your notebook.
- Plot your results using a graph in your notebook. If your notebook does not have a grid (is not quad-ruled or graph-ruled), you must use graph paper or a computer graphing program such as Excel (see Creating Graphs using Excel)
A) Be sure that you put the independent variable on the X axis.
B) A line graph is appropriate for continuous data. A bar graph is more appropriate for data that fall into categories with no particular order to the categories. In this case, time is continuous. We measured it in five increments but it is possible to have measurements between the five.
- Did the rate of gain appear to be constant? You can answer this question by seeing if the graph is a straight line.
- What do you predict would happen to the bag after one day?
Osmosis in Potato Cells
- Create a hypothesis for the experiment described below.
- Cut two strips of potato about the size of a French fry (approximately 5 mm X 5 mm X 50 mm).
- Put one of the strips in a small beaker that contains enough 10% NaCl to cover the potato.
- Put the other strip in a beaker that contains enough deionized water to cover the potato.
- Remove the strips from the beakers after about 60 minutes and examine the potatoes. Is one of them limp? Is one firm? Record your observations in your notebook.
- Explain your observations. Be to mention where the concentration of water molecules is greater (and salt is less) and where the concentration of water molecules is less (salt is greater).
Plasmolysis in Elodea
- Create a hypothesis for the experiment described below.
- Obtain a leaf of Elodea and place it on a blank microscope slide.
- Dry it with a paper, add a drop or two of 10% NaCl, and then cover it with a cover slip.
- Observe the cells under scanning and low power immediately after you prepare the slide.
- Let the slide sit for 10 minutes and observe the cells again. It may be helpful to use a brighter light to view the cells. As the cell shrinks, the chloroplasts will appear to clump together.
- Describe what happened to the cells.
- Why did this happen? To help you answer this question, consider where the concentration of water molecules is greatest and where it is least.